Hormones alter immunity, driving sex bias in cancer
2022-06-09
Cancer is a disease that can strike in anyone, but often plays favorites based on a wide variety of factors, including biological sex (as defined by chromosomal and hormonal patterns). While one might expect that this bias is only relevant in cancers that affect the reproductive system, (like cervical cancer or prostate cancer), cancer in non-reproductive organs (like the lungs or bladder) also shows strong sex bias, often developing more frequently and more aggressively in males, and contributing to higher mortality.
Thus far, no behavioral, physical, or genetic differences between male and female patients have been able to explain this phenomenon. However, with more recent research efforts highlighting the important role of the immune system in controlling cancer, researchers at the lab of Zihai Li, PhD at The Ohio State University decided to investigate whether differences in immune responses in males and females might be to blame for sex bias in cancer. Working towards his PhD thesis around cancer immunology, then-graduate student Hyunwoo “Tony” Kwon, PhD took on the lead role in the project, the results of which were recently published in the journal Science Immunology.
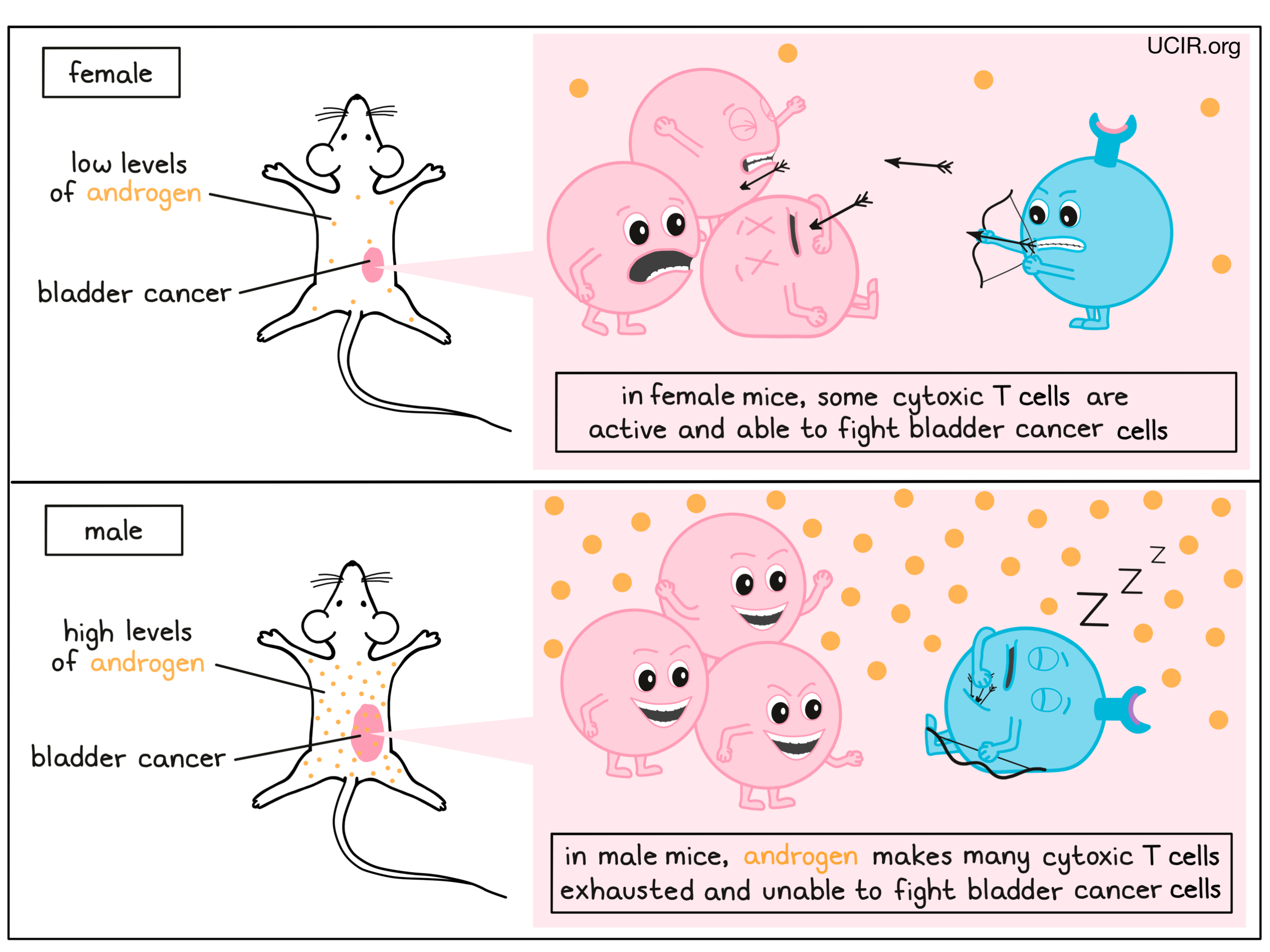
For their investigation, the researchers focused on bladder cancer, which is both more common and more deadly in male patients. This fact was also found to be true in mice exposed to a bladder cancer-causing carcinogen, and in mice that were directly injected with bladder cancer cells. To figure out which immune cells might influence this effect, the researchers eliminated different immune cell types one at a time. When an immune cell group called cytotoxic T cells, which happen to be well known for their role in fighting cancer (both naturally and in the context of immune-enhancing cancer therapies), were depleted in mice, the bladder cancers grew equally in males and females. The fact that these cells contributed different levels of immunity against cancer in male and female mice indicated to the researchers that something about these cells is different between the sexes, and contributes to sex bias.
Looking more closely at possible differences between these cells in mice of different sexes, the team looked at T cells from within the tumor mass, and found that in females, these cells were activated and ready to fight, while in males they were either exhausted or showed signs that they soon would be. Further, when T cells were set up to directly fight cancer cells in mice, the T cells from females were far better at killing the cancer cells than the T cells from males, confirming the important influence of these cells on different outcomes in cancer.
Digging into what might be driving such differences, the researchers investigated the influence of genetic and hormonal differences between males and females on immune characteristics. Using an interesting set of mouse models that includes typical XX females and XY males alongside genetically sex-reversed XY females and XX males, the researchers found that cancer was more aggressive in mice that presented as males (anatomically and hormonally), regardless of which chromosomes were present. Looking at the hormonal aspect, the researchers found that bladder tumors grew more aggressively in mice exposed to high levels of testosterone – a hormone that is typically produced at much higher levels in males than females. Testosterone falls under a category of hormones called androgens, which can interact directly with androgen receptors in the T cells. This interaction between androgens and androgen receptors was found to lead to a chain reaction of signals and changes within the T cells, and was responsible for the differential behavior of the T cells, and for driving them towards a dysfunctional state in males.
Testing whether interfering with this chain of signals could be used to improve immune responses to cancer, the researchers found that removing the source of the testosterone (by surgical castration of male mice) or by blocking testosterone from interacting with the receptors in the T cells could effectively reduce T cell exhaustion, allowing the cells to instead develop cancer-fighting functions and retain them for longer. Further, interfering with androgen signaling – a drug-based strategy that is sometimes used to treat prostate cancer – during treatment with an anti-PD-1 checkpoint inhibitor (e.g. Keytruda, Opdivo, or Libtayo) – one of the most widely used cancer immunotherapies in the clinic – improved outcomes in mice, suggesting that information uncovered in this new research could have practical applications that may eventually benefit patients with cancer, after appropriate clinical investigation.
Overall, this research shows that different levels of hormones in males and females affects the ability of the immune system to protect against cancer, and contributes to the sex bias that is frequently observed in cancer. Understanding this mechanism can help to ensure that patients get the best care possible, and can help lead to new treatments that target the immune system and could help to reduce the incidence and severity of cancer, particularly in males.
By Lauren Hitchings
References:
- Original paper - Androgen conspires with the CD8+ T cell exhaustion program and contributes to sex bias in cancer
- ACIR feature article - Hormone-driven immune exhaustion fuels sex bias in cancer