How is this drug name pronounced?
Fam-trastuzumab deruxtecan: fam-tras-TOO-zoo-mab DEH-rux-TEE-kan
Enhertu: en-HER-too
What cancer(s) does this drug treat?
Enhertu is approved for:
Breast cancer
Non-small cell lung cancer
Stomach cancer
Advanced breast cancer
Enhertu is approved for:
-
Patients with advanced breast cancer that tests positive for the HER2 molecule and either cannot be removed by surgery or has spread to other parts of the body, and
- who have previously received at least one prior anti-HER2-based treatment after their disease had spread, OR in combination with surgery, but the disease came back during, or within six months of completing the therapy.
-
Patients with advanced breast cancer that tests positive for low amounts of the HER2 molecule and either cannot be removed by surgery or has spread to other parts of the body, and
- who have received chemotherapy after their disease had spread, OR
- whose disease came back during or within six months of completing chemotherapy after surgery.
Advanced non-small cell lung cancer
Enhertu is approved for:
- Patients with previously treated non-small cell lung cancer that tests positive for activating mutations in the HER2 molecule and either cannot be removed by surgery or has spread to other parts of the body.
Advanced stomach cancer
Enhertu is approved for:
- Patients with advanced gastric adenocarcinoma (stomach cancer) or gastroesophageal junction adenocarcinoma (cancer of the area where the stomach meets the esophagus) that tests positive for the HER2 molecule and has grown or spread to other parts of the body, and who have previously received treatment with trastuzumab (Herceptin) for their advanced disease.
Limitations of use:
Age: The safety and efficacy of Enhertu in patients under 18 years of age have not been established.
Fertility/Pregnancy/Breastfeeding: Enhertu may impair fertility in men. Enhertu can result in the death of the fetus and can cause birth defects. Women should not start Enhertu treatment when pregnant and should use effective contraception to prevent pregnancy during treatment with Enhertu and for at least seven months after the last dose of Enhertu. Men should use effective contraception during treatment with Enhertu and for at least four months after the last dose of Enhertu. Enhertu may cause serious adverse reactions to the breastfed child. Women are advised not to breastfeed during treatment with Enhertu and for at least seven months after the last dose of Enhertu.
What type of immunotherapy is this?
- Antibody-drug conjugate
How does this drug work?
- Target: HER2
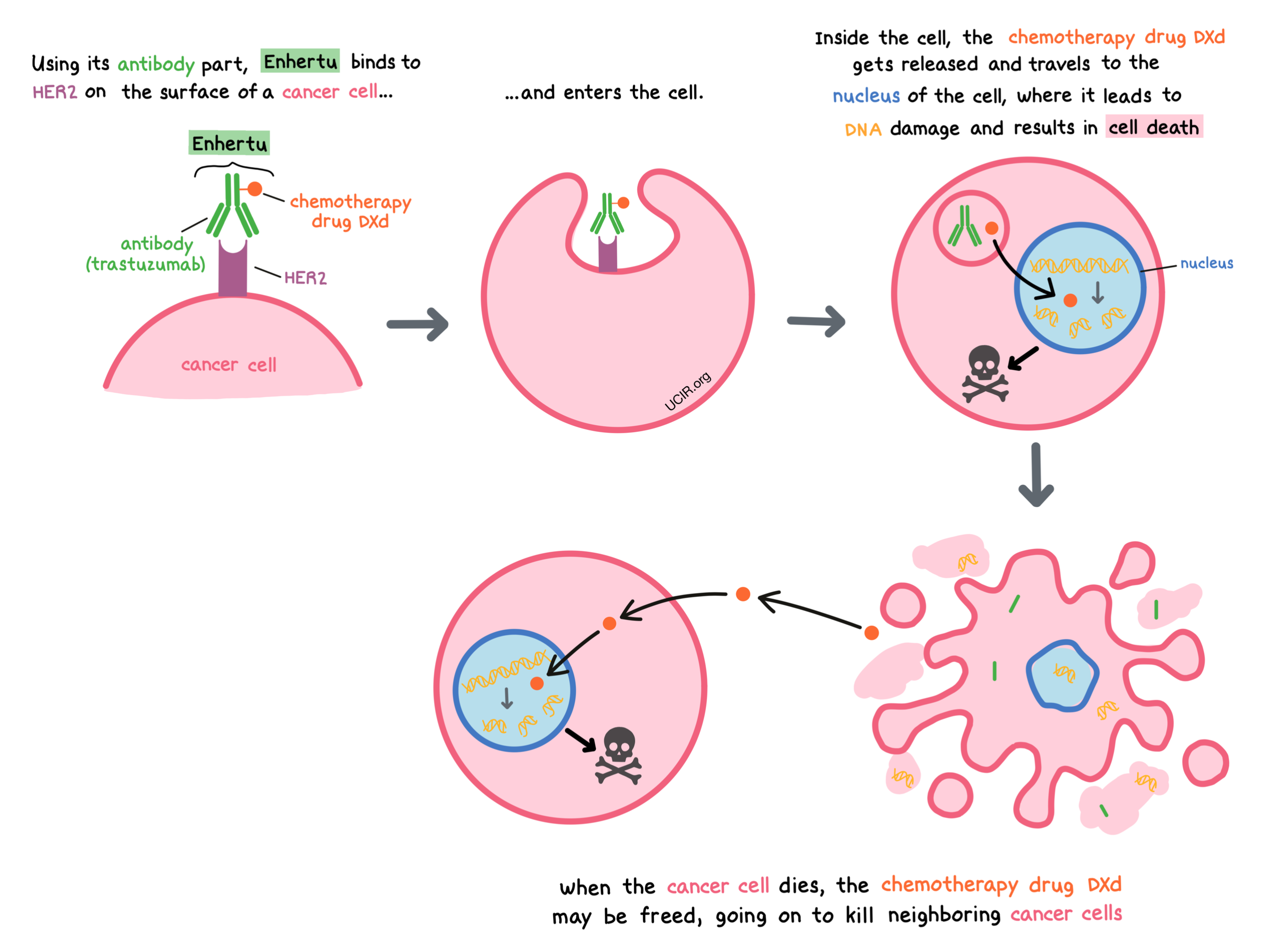
Enhertu is a medicine that consists of two parts that are connected: an antibody called trastuzumab that attaches to a molecule called HER2 on the surface of cancer cells, and a chemotherapy drug called DXd. HER2 is a protein present on the surface of different types of healthy cells, and it plays a role in regulating the multiplication and survival of cells. HER2 is also found in higher quantities on the surface of some breast cancer cells.
By targeting HER2, Enhertu is designed to minimize harm to normal, healthy cells and to bring the chemotherapy directly to the cancer cells. When Enhertu binds to HER2, it can enter the cell it is bound to. Inside the cell, the chemotherapy part of Enhertu, known as DXd, is released. DXd travels to the nucleus of the cell, where it causes DNA damage, and leads to cell death. Free DXd can then also enter surrounding cancer cells that may have lower amounts of HER2 on their surface and may not be directly targeted by Enhertu.
How is this drug given to the patient?
Enhertu is administered via a tube into a vein (intravenous infusion, or I.V.) every 3 weeks. The first infusion is administered over 90 minutes. If the first infusion is well tolerated, subsequent infusions are administered over 30 minutes.
Before the administration of Enhertu, patients may receive mediation to prevent nausea and vomiting.
What are the observed clinical results?
For:
Advanced breast cancer
Advanced non-small cell lung cancer
Advanced gastric cancer
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies.
Advanced breast cancer
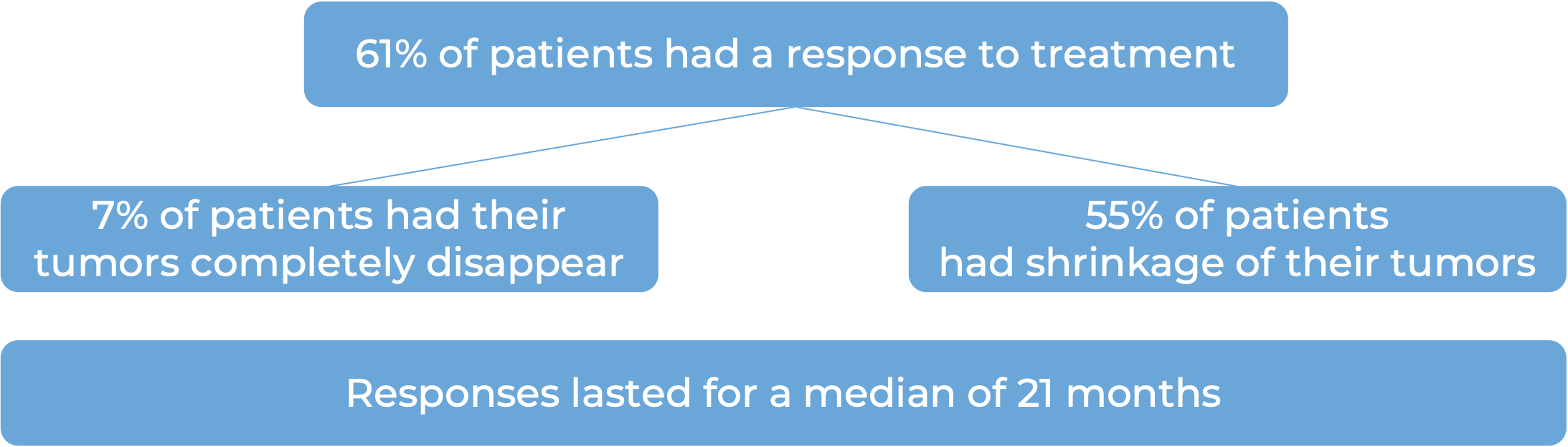
In a clinical trial, 184 patients with advanced breast cancer that could not be removed by surgery and/or had spread to other parts of the body, and that tested positive for the HER2 molecule, who had previously received two or more anti-HER2-based treatments, were treated with Enhertu. At a median follow-up of 11 months, 60% of patients responded to treatment, including 4% whose tumors went away completely and 56% who saw their tumors shrink. Patients continued to respond for a median of 15 months.
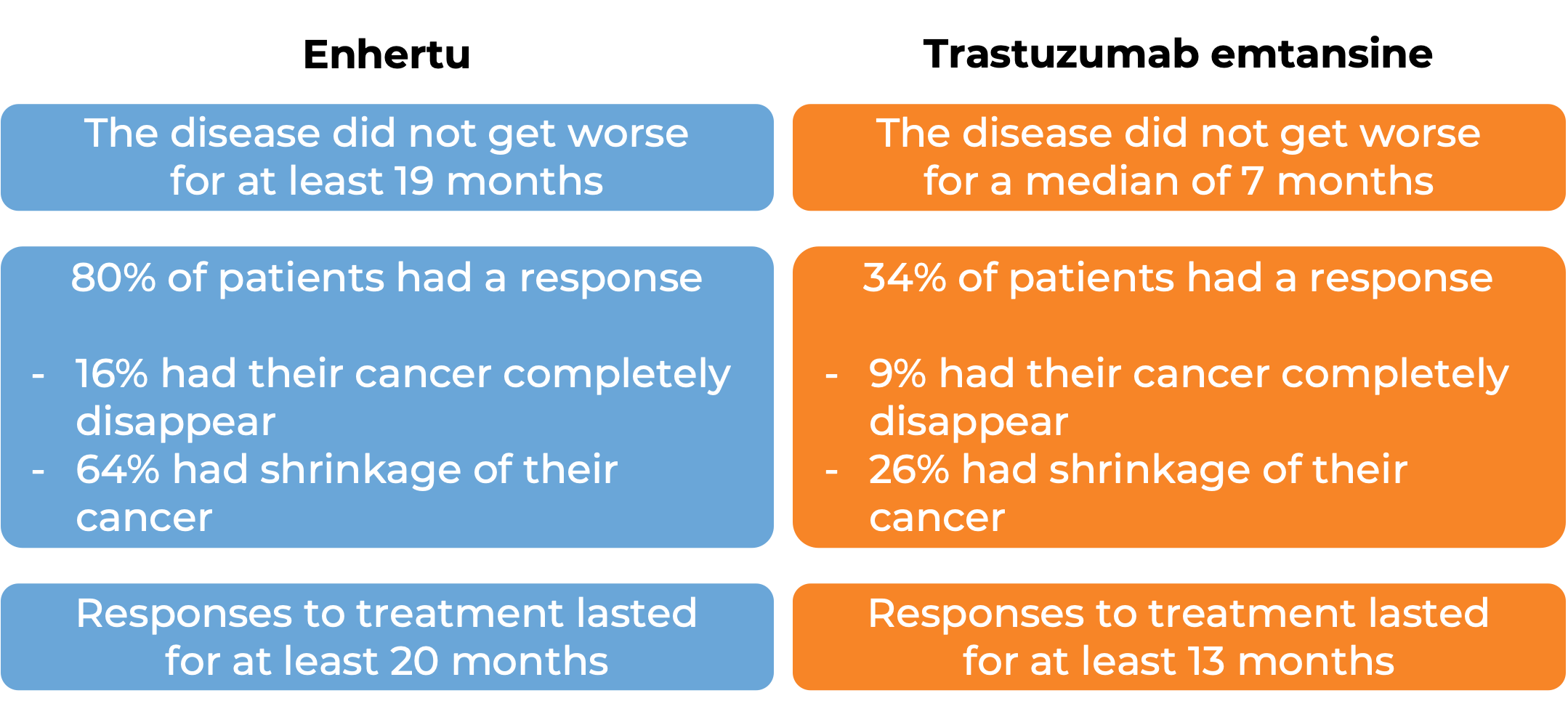
In another clinical trial, 524 patients with breast cancer that tested positive for the HER2 molecule and could not be removed by surgery and/or has spread to other parts of the body, and who had received trastuzumab (e.g., Herceptin) and taxane chemotherapy or whose disease came back during or within 6 months after completing chemotherapy treatment after surgery, were treated with Enhertu or ado-trastuzumab emtansine (Kadcyla). At a median follow-up of 15 to 16 months:
(For the definition of “median”, click HERE.)
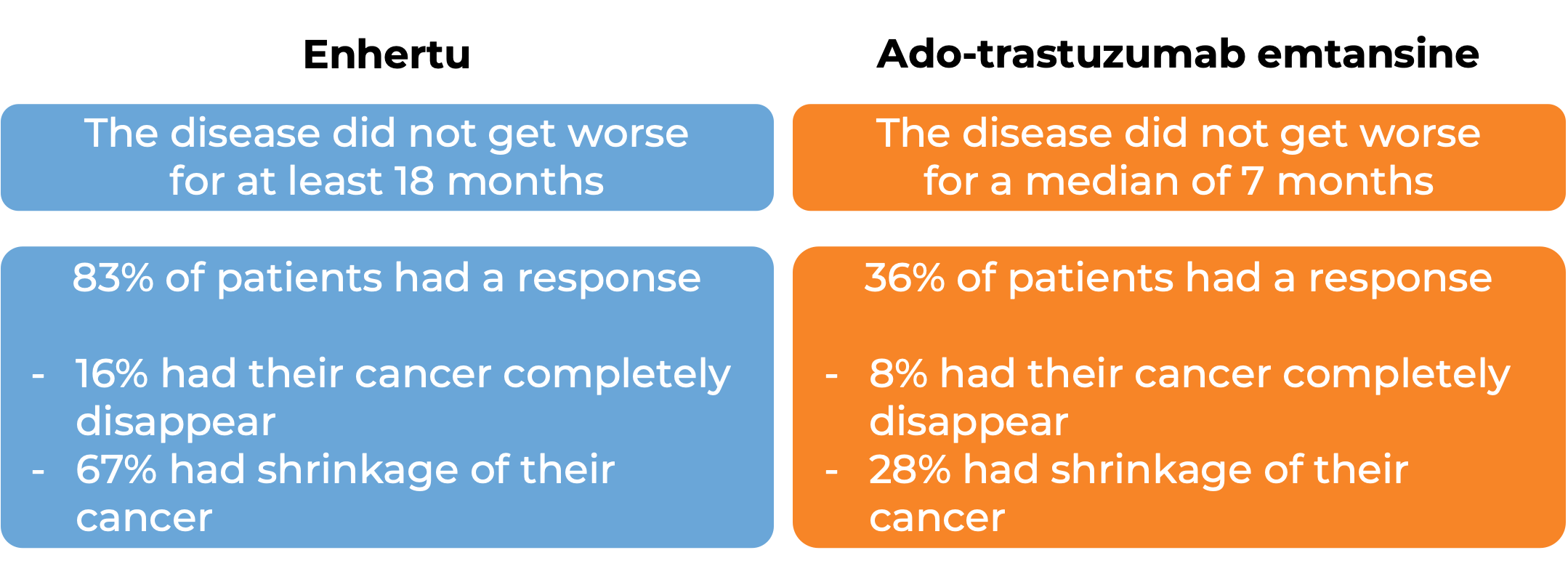
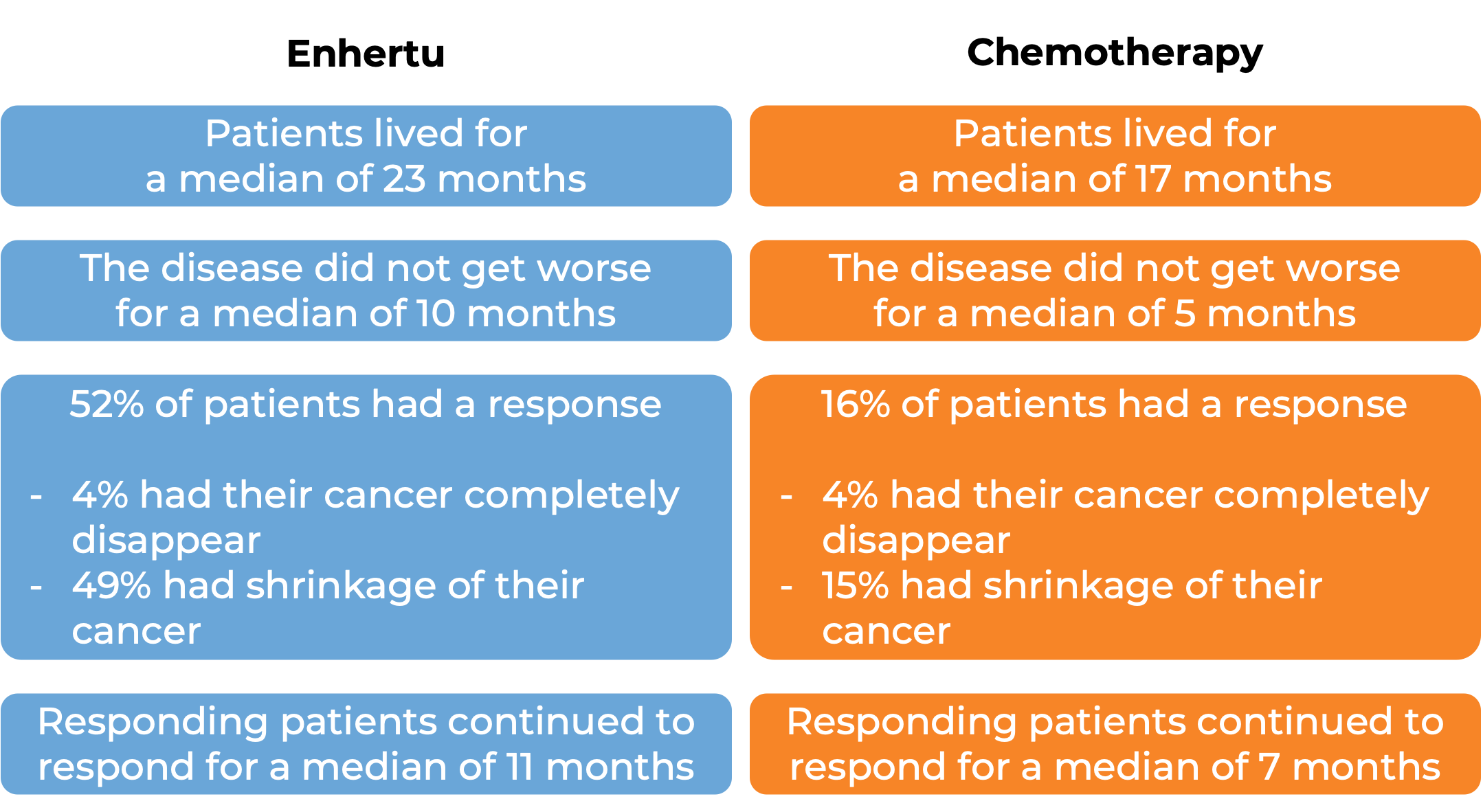
In another clinical trial, 557 patients with previously treated advanced breast cancer that tested positive for low levels of the HER2 molecule and could not be removed by surgery and/or had spread to other parts of the body, were either treated with Enhertu or chemotherapy. At a median follow-up of 18 months:
Advanced non-small cell lung cancer
In a clinical trial, 52 patients with previously treated non-small cell lung cancer that tested positive for activating mutations in the HER2 molecule and either could be removed by surgery or had spread to other parts of the body were treated with Enherty. 58% of patients responded to treatment, including 2% whose tumors went away completely and 56% who saw their tumors shrink. Patients continued to respond for a median of 8 months.
Advanced stomach cancer
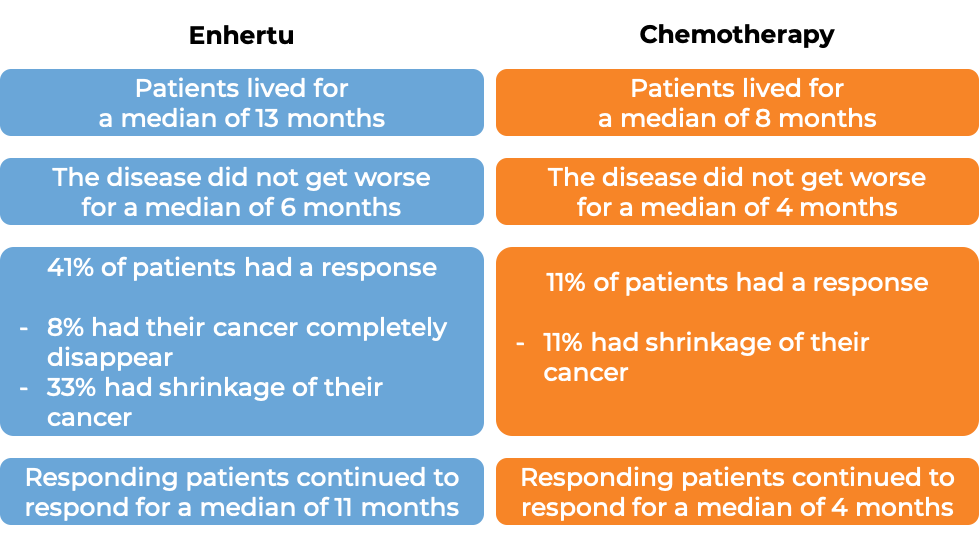
In a clinical trial, 188 patients with previously treated advanced gastric or gastroesophageal junction adenocarcinoma that tested positive for the HER2 molecule and had grown or spread to other parts of the body, were treated with Enhertu or the physician’s choice of either irinotecan or paclitaxel chemotherapy.
(For the definition of “median”, click HERE.)
What are the side effects?
The most common side effects of Enhertu include tiredness, nausea, vomiting, constipation, diarrhea, decreased appetite, cough, hair loss, muscle or bone pain, low red blood cell count, low white blood cell count, and low platelet count.
Enhertu can cause side effects that can become serious or life-threatening, and may lead to death. Some of the serious side effects related to Enhertu include severe problems with the lungs and heart, and severe low white blood cell count (which can increase the risk of infection).
Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects. For a more complete list of possible side effects, see the full prescribing information.
Additional information
Manufacturer
Daiichi-Sankyo and AstraZeneca
Approval
FDA and EMA
Links to drug websites
Other references
- Tratuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. S. Modi et al. The New England Journal of Medicine (2020)
- Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. J Cortes et al. The New England Journal of Medicine (2022)
- Tratuzumab Deruxtecan in Previously Treated HER-2 Low Advanced Breast Cancer. S. Modi et al. The New England Journal of Medicine (2022)
Last updated on January 27, 2023