How is the drug name pronounced?
Amtagvi: ahm-TAG-vee
Lifileucel: LIH-fee-LOO-sel
What cancer(s) does this drug treat?
Melanoma
Amtagvi is approved for:
- Patients with melanoma that cannot be removed by surgery or has spread to other parts of the body, and who have previously been treated with a PD-1-blocking antibody and, if the patient tested positive for the BRAF V600 mutation, with a BRAF inhibitor and optionally, a MEK inhibitor.
Limitations of Use
Age: The safety and efficacy of Amtagvi in patients under 18 years of age have not been established.
Pregnancy/Breastfeeding: The risks associated with Amtagvi during pregnancy are not known and cannot be ruled out. Amtagvi is not recommended for use during pregnancy. The risks associated with Amtagvi during breastfeeding are not known and cannot be ruled out. Patients are advised to weigh the benefits of breastfeeding and the mother’s clinical need for treatment with Amtagvi and any potential risks of side effects.
Corticosteroids: Corticosteroids should not be administered shortly before Amtagvi treatment, as they may limit the activity of Amtagvi.
What type of immunotherapy is this?
How does this drug work?
Target:
- Various cancer antigens and neoantigens recognized by the patient’s own T cells
Amtagvi is made from the patient’s own lymphocytes, including T cells, which have strong and highly specific cancer-killing abilities. In order to make Amtagvi, a portion of the patient’s tumor is removed, and any lymphocytes infiltrating the tumor (TIL) are collected. These cells are then cultured and multiplied in a laboratory setting, generating an army of billions of T cells, many of which are likely to recognize cancer cells. The T cells are then delivered back to the patient as a living drug, where they continue to multiply, and where they are able to recognize and kill cancer cells, without harming healthy cells.
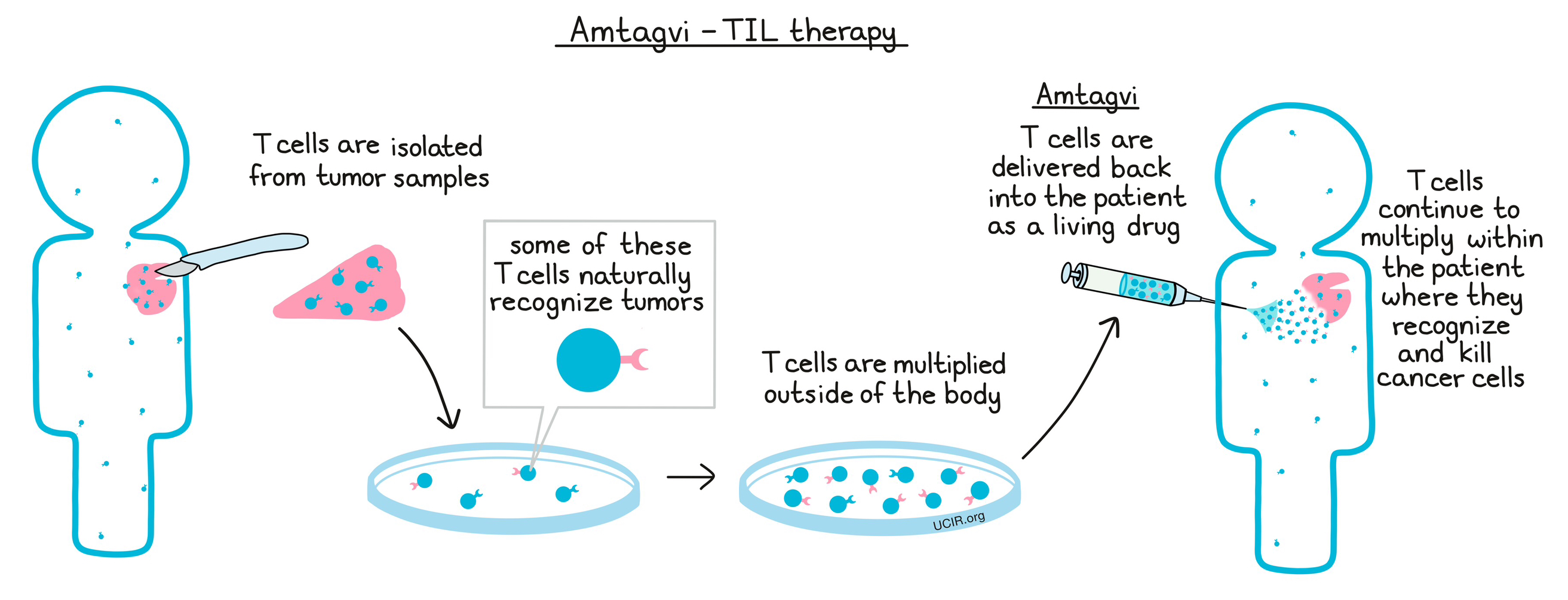
How is this drug given to the patient?
Treatment with Amtagvi requires a hospital stay. Before receiving Amtagvi, patients are treated with cyclophosphamide chemotherapy for 2 days and then with fludarabine chemotherapy for 5 days to reduce the number of white blood cells in their system. The reduction in the patient’s white blood cell count gives the lymphocytes in Amtagvi enough space to multiply, and provides them with the resources needed to survive longer in the patient. Amtagvi is given 1-4 days after the last dose of chemotherapy. About 30 to 60 minutes before receiving Amtagvi, patients receive a fever reducer (acetaminophen) and an antihistamine (e.g., diphenhydramine) to reduce the chance of reactions to the infusion. Patients receive Amtagvi through an intravenous (i.v.) infusion, which usually takes less than 1 and a half hours. After infusion, patients are treated with IL-2 through an i.v. infusion every 8-12 hours for up to 6 doses, to promote expansion of Amtagvi within the patient.
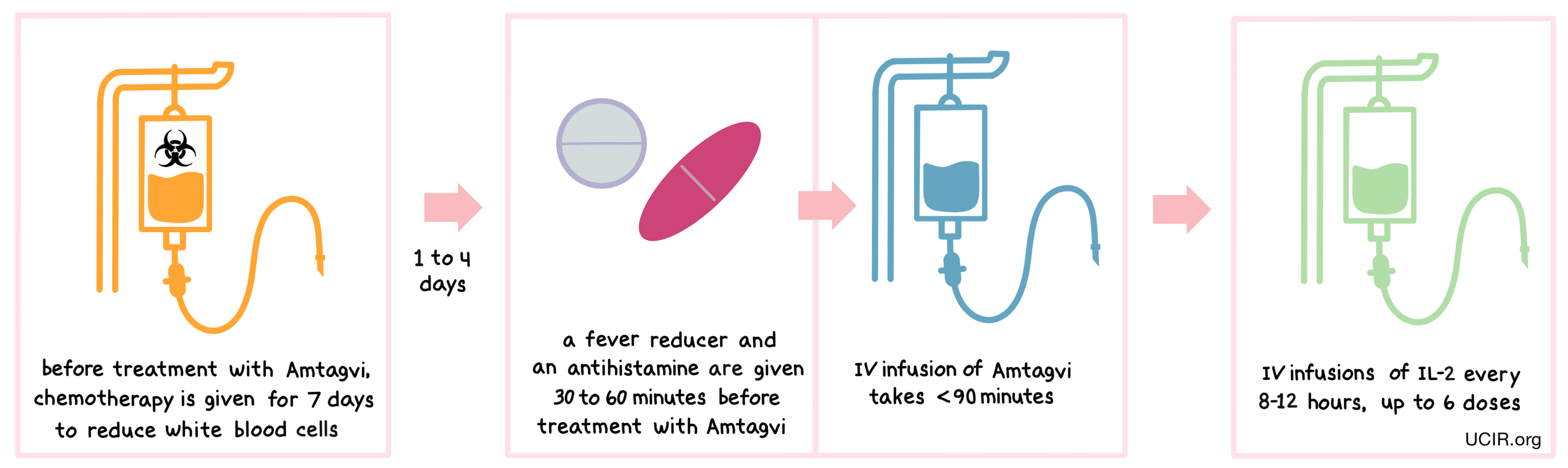
Patients can leave the hospital after finishing their IL-2 treatments, but should plan to stay within 2 hours of the treatment location for several weeks after receiving Amtagvi for follow-up appointments and to address any potential side effects that may occur.
What are the observed clinical results?
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies.
Melanoma
In a clinical trial, 89 patients with melanoma that could not be removed by surgery or had spread to other parts of the body, and who had had been previously been treated with a PD-1-blocking antibody and, if applicable, BRAF (and MEK) inhibitors, were treated with Amtagvi. Among 73 patients who were treated with what was considered an effective dose:
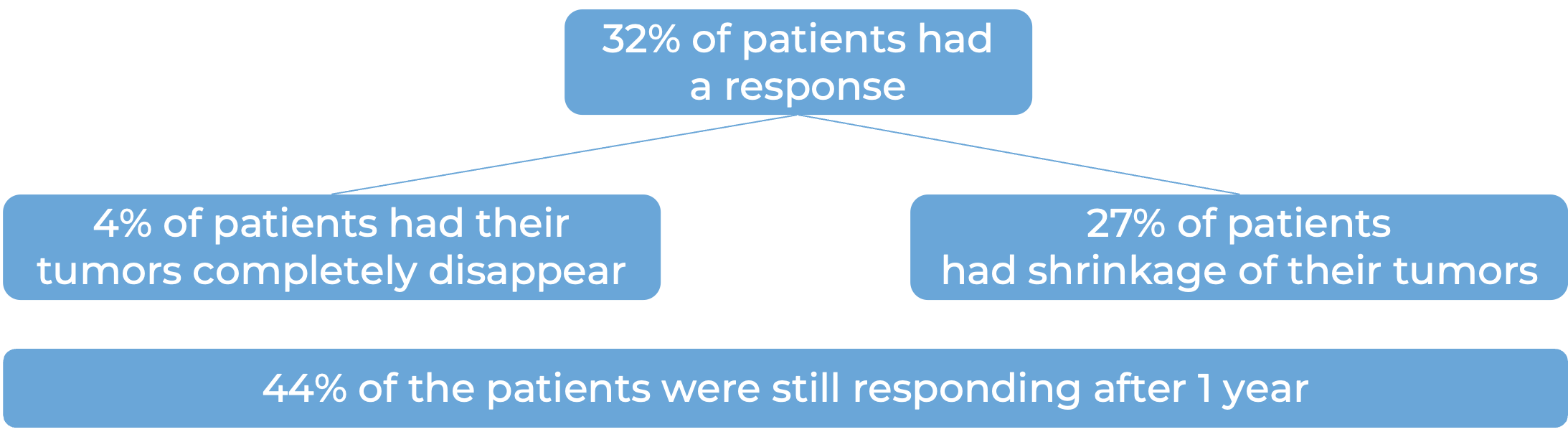
Another dataset from 153 patients treated with Amtagvi delivered similar results: 31% had a response to treatment, with 5% having their tumors completely disappear, and 26% having partial shrinkage of their tumors. Among patients who had a response, 54% were still responding after 1 year.
What are the potential side effects?
The most common side effects of Amtagvi include chills, fever, fatigue, increased heart rate, diarrhea, reduced counts of neutrophils in the blood, swelling, rash, low blood pressure, hair loss, infection, low levels of oxygen in the blood, and difficulty getting a full breath of air.
Some side effects, such as prolonged reduction in blood cells, infections, internal organ bleeding, kidney failure, respiratory failure, irregular heart rhythms, fluid buildup, liver injury, and bone marrow failure could be severe and life-threatening. Patients may also experience reactions to the infusion.
Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects. For a more complete list of possible side effects, see the full prescribing information.
Additional Information
Manufacturer
WuXi Advanced Therapeutics, Inc. Iovance Biotherapeutics Manufacturing, LLC
Approval
FDA
Links to drug website
Last updated: July 1, 2025


