How is this drug name pronounced?
Loncastuximab tesirine: Lon- kas-TUX-i-mab TES-ir-een
Zynlonta: Zin-LON-ta
What cancer(s) does this drug treat?
Large B-cell Lymphoma
Zynlonta is approved for:
- Adult patients with large B-cell lymphoma, including diffuse large B-cell lymphoma (DLBCL) and high grade B-cell lymphoma, whose cancer did not respond (relapsed or refractory) to or came back after two or more previous treatments.
Limitations of use:
Age: The safety and efficacy of Zynlonta in patients under 18 years of age have not been established.
Fertility/Pregnancy/Breastfeeding: Zynlonta may cause permanent infertility in male patients. Zynlonta can cause harm to a fetus and is not recommended for use during pregnancy. Women are advised to use contraception during treatment with Zynlonta and for ten months after the last dose of Zynlonta. Men should use contraceptive methods during treatment with Zynlonta and for seven months after the last dose of Zynlonta. The risks associated with Zynlonta during breastfeeding are not known and cannot be ruled out. Due to the potential for serious reactions in the breastfed child, women are advised not to breastfeed during treatment and for at least three months after the last dose of Zynlonta.
What type of immunotherapy is this?
- Antibody–drug conjugate
How does this drug work?
- Target: CD19
Zynlonta is a medicine that consists of two parts that are connected: an antibody that attaches to a protein molecule called CD19 on the surface of B cells, and a chemotherapy drug called teserine that can kill cancer cells.
When Zynlonta attaches to CD19 and enters the cancerous B cell, the cell-killing drug part of Zynlonta, teserine, is released, becomes activated, and travels to the nucleus of the cell. In the nucleus, teserine binds to the cancer cell’s DNA, causing the cell to die.
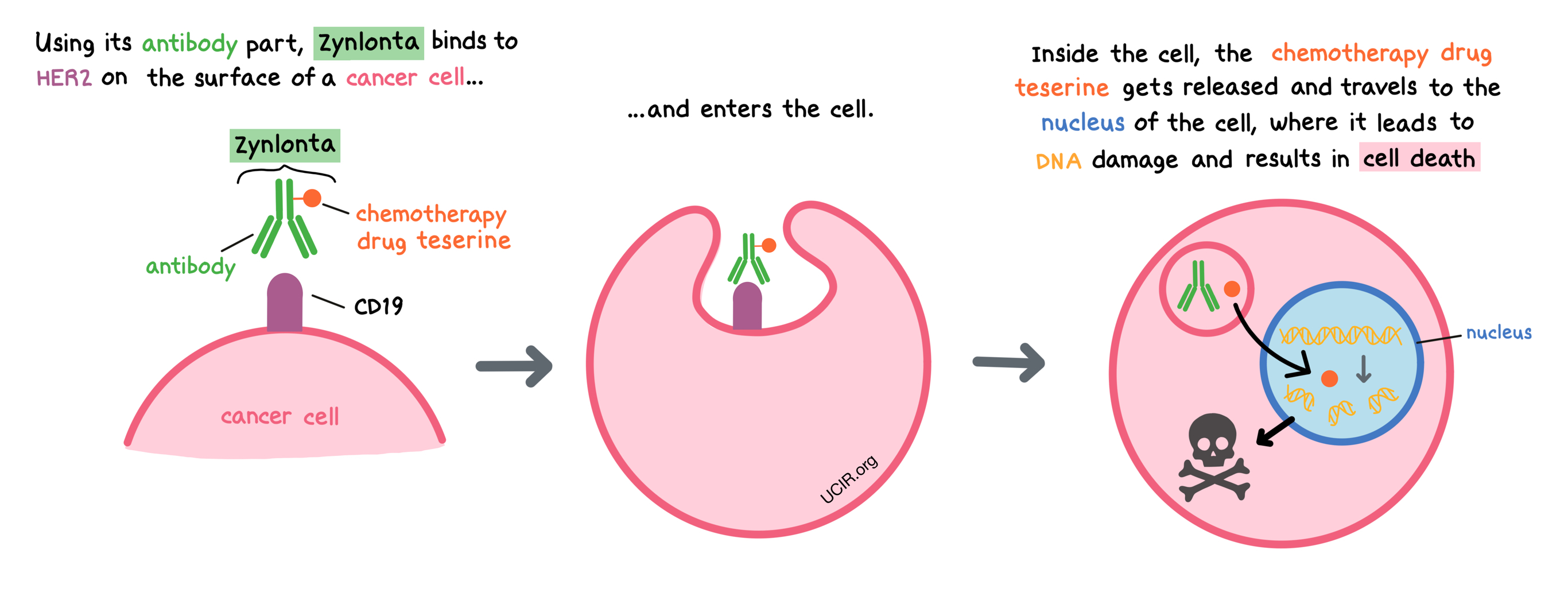
CD19 can also be found on the surface of all normal B cells, where it plays a role in cellular activation. In addition to attacking cancer cells, Zynlonta can also attack and kill healthy B cells.
How is this drug given to patients?
Zynlonta is administered via a tube into a vein (intravenous infusion, or i.v.) over the course of 30 minutes. Zynlonta is given to the patient every 3 weeks.
The day before and for two days after Zynlonta infusion, patients are given dexamethasone via a tube in the vein to reduce the risk of reactions related to the infusion.
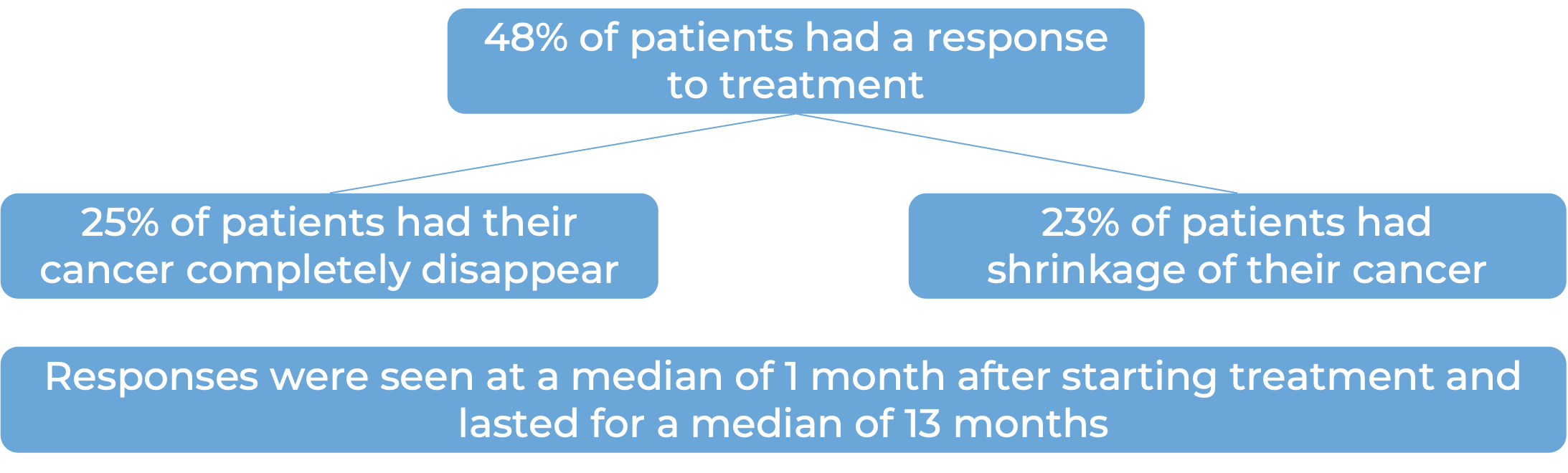
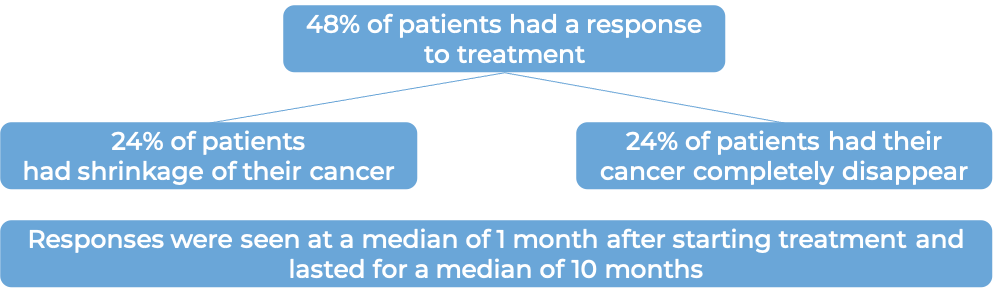
What are the observed clinical results?
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Large B-cell lymphoma
In a clinical trial, 145 patients with diffuse large B-cell lymphoma (DLBCL) that had come back or did not respond after two or more previous treatments, were treated with Zynlonta. At a median follow-up of 7 months:
(For the definition of “median” click HERE.)
What are the side effects?
The most common side effects of Zynlonta are fatigue; nausea; swelling of hands and feet; muscle, bone, or joint pain; abnormal blood tests, including an increase in blood sugar (hyperglycemia); and skin rashes. Exposure to sunlight could worsen skin reactions, and patients are advised to limit sun exposure and wear sunscreen and hats.
Zynlonta may also cause serious side effects, including fluid retention and buildup of extra fluid in the abdomen or around the lungs or heart, low white blood cell count (neutropenia), low platelet count, low red blood cell count (anemia), and severe skin reactions. Serious, life-threatening infections may occur, including pneumonia or sepsis.
Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects. For a more complete list of possible side effects, see the full prescribing information.
Additional Information:
Manufacturer:
ADC Therapeutics
Approval:
FDA
Links to drug websites:
Last updated January 26, 2023