How is this drug name pronounced?
Mosunetuzumab: moh-SUN-eh-TOO-zoo-mab
Lunsumio: lun-SUM-ee-oh
What cancer(s) does this drug treat?
Follicular lymphoma
Lunsumio is approved for:
- Patients with follicular lymphoma who have received at least two prior treatments for their disease, but whose cancer either did not respond to treatment or has since come back.
Limitations of use
Limitations: Lunsumio should only be administered in a treatment center and by adequately trained healthcare professionals who can ensure proper monitoring and immediate management of side effects associated with the drug.
Age: The safety and efficacy of Lunsumio in patients under 18 years of age have not been established.
Pregnancy/Breastfeeding: Lunsumio is not recommended for use during pregnancy due to potential for harm to the fetus. Patients are advised to use contraception during treatment with Lunsumio and for at least 3 months after the last dose of Lunsumio. The risks associated with Lunsumio during breastfeeding are not known and cannot be ruled out. Due to the potential for adverse reactions in the breastfed child, patients are advised not to breastfeed during treatment with Lunsumio and for at least 3 months after the last dose of Lunsumio.
Interactions with other drugs: Dose adjustments may become necessary for patients treated with CYP450 substrates (e.g., warfarin, cyclosporin, theophylline) at the start of treatment with Lunsumio.
What type of immunotherapy is this?
How does this drug work?
Targets:
- CD20 on B cells
- CD3 receptor on T cells
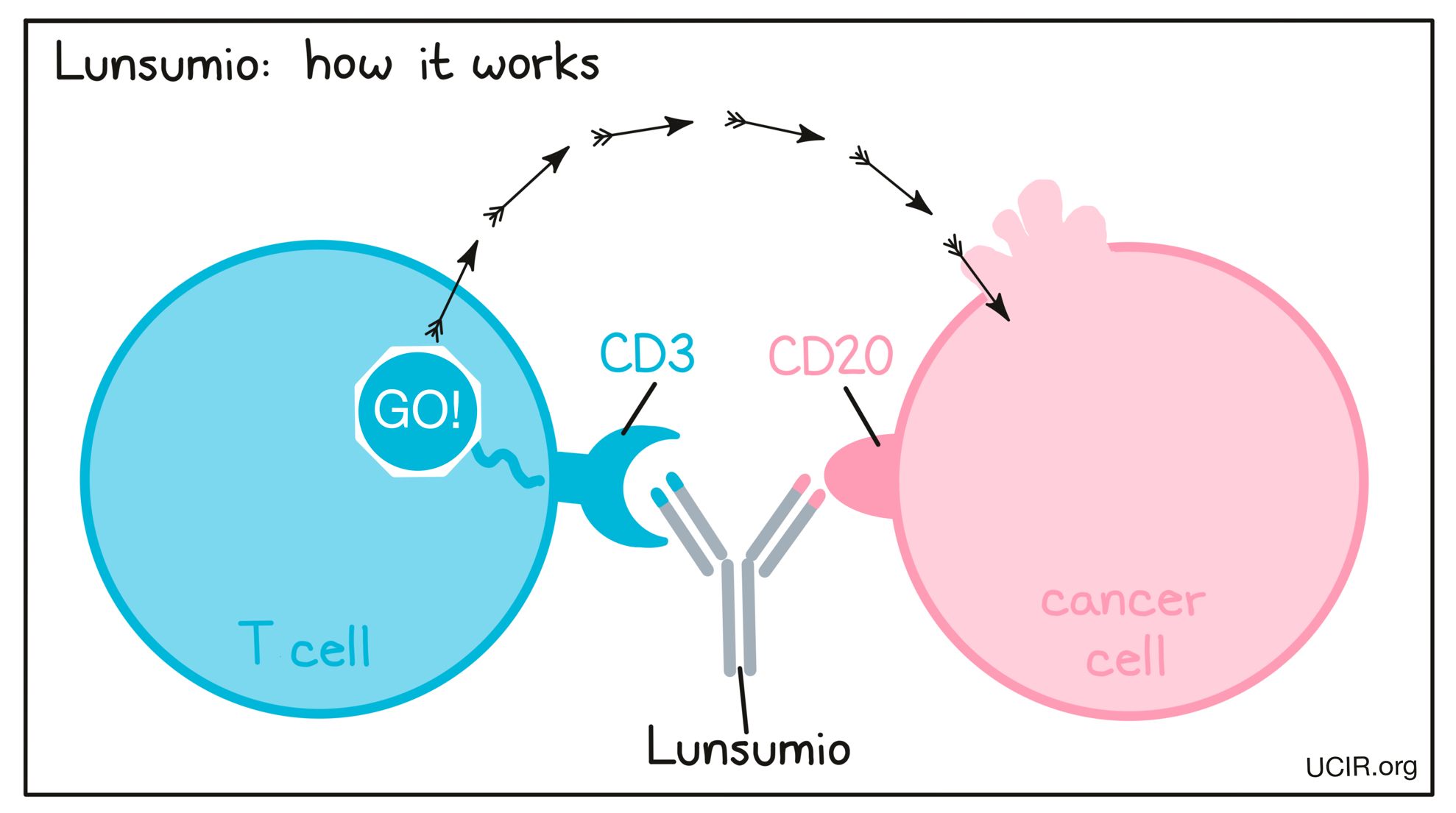
Lunsumio is a “bispecific antibody”. Antibodies are molecules that can bind to a particular target molecule. Bispecific antibodies are artificially made in the laboratory and can bind to two different targets at the same time. Lunsumio binds to:
- a molecule called CD20 on the surface of follicular lymphoma cells and some healthy B cells. CD20 is present at much higher quantities on the surface of cancer cells than normal healthy cells.
- a molecule called CD3 on the surface of T cells – the primary immune cells involved in killing cancer cells. CD3 is part of the T cell receptor, which is critical to the function of the T cell and is involved in stimulating the T cell to become active.
Because Lunsumio can bind to one molecule on follicular lymphoma cells and another on T cells at the same time, it acts as a bridge and keeps the T cell in close contact with the cancer cell. By binding CD3 on the T cell, Lunsumio also stimulates the T cell to become activated and kill the cell it is bound to. Bispecific antibodies that direct T cells to kill cancer cells by binding to both cells at the same time are known as bispecific T cell engagers (BiTEs).
How is the drug given to the patient?
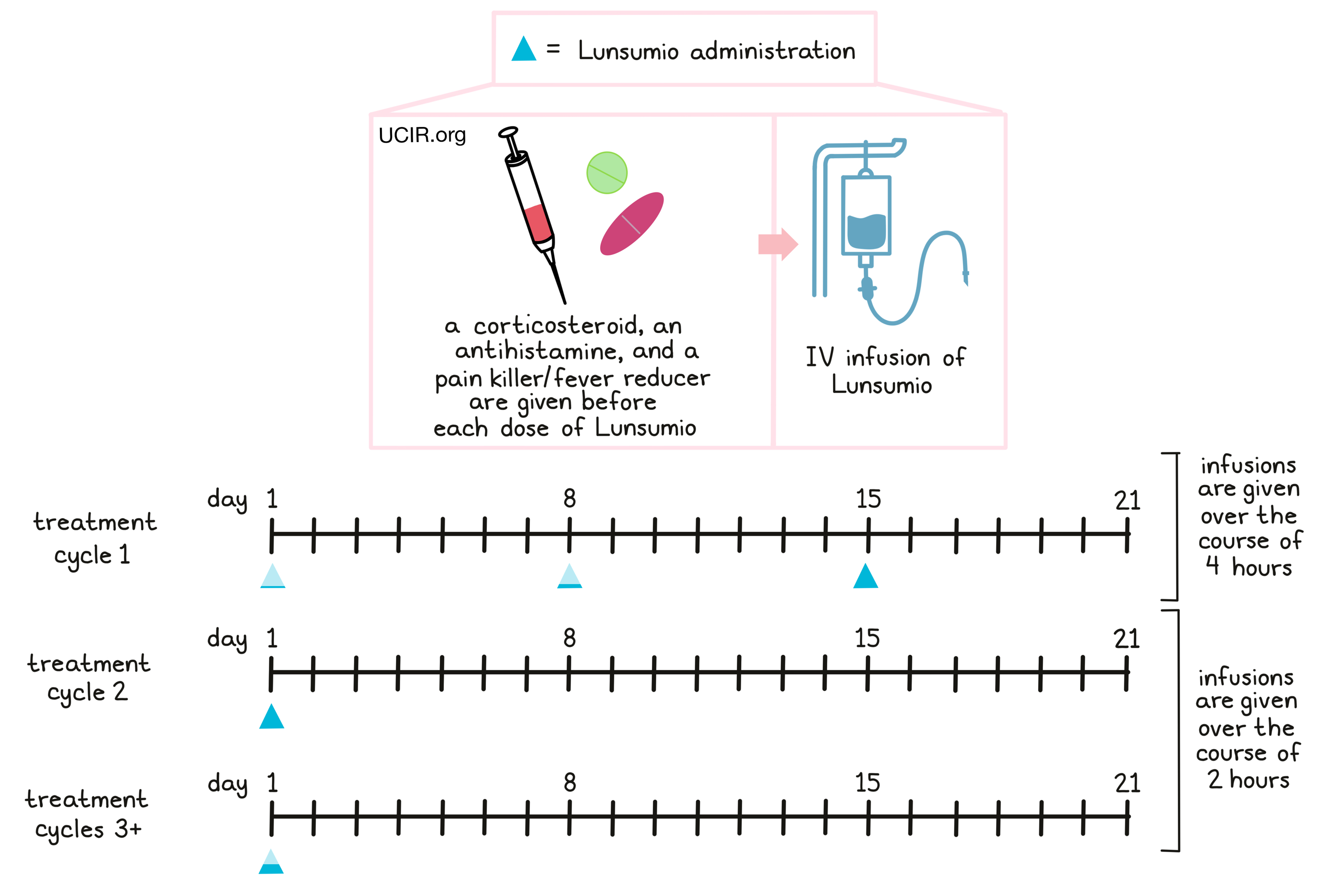
About 1 hour to 30 minutes prior to receiving each dose of Lunsumio in treatment cycle 1 and 2 and if needed in subsequent treatment cycles, patients receive several medications to help reduce the risk of cytokine release syndrome:
- A corticosteroid (dexamethasone or methylprednisolone)
- An antihistamine (diphenhydramine)
- A painkiller and fever reducer (acetaminophen)
Patients should be well hydrated before they receive Lunsumio, which is administered through a tube in the vein (intravenous (IV) infusion). During the first treatment cycle (a 21-day period), each dose of Lunsumio is delivered over the course of at least four hours. A low dose of Lunsumio is administered on day 1, a second, slightly higher dose of Lunsumio is given on day 8, and the first full dose of Lunsumio is administered on day 15 of treatment cycle 1. During the second treatment cycle, a full dose of Lunsumio is administered on day 1 over the course of 2 hours. For every subsequent treatment cycle, half of the full dose of Lunsumio is given on day 1 over the course of 2 hours. Before and periodically during the treatment, blood tests are performed to monitor for side effects.
What are the observed clinical results?
It is important to keep in mind that each patient’s outcome is individual and may be different from the results found in the clinical studies. In addition, immunotherapy can sometimes take several months to yield an observable treatment response.
Follicular lymphoma
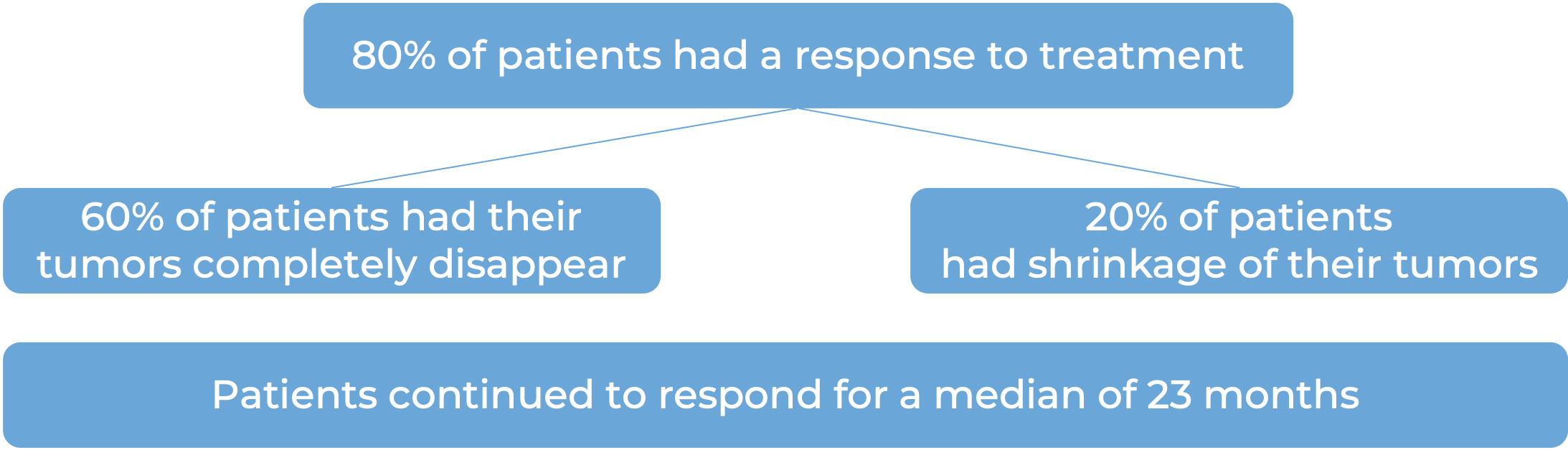
In a clinical study, 90 patients with follicular lymphoma who had received at least two prior treatments for their disease, but whose cancer either did not respond to treatment or had since come back, were treated with Lunsumio. At a median follow-up of 25 months:
What are the potential side effects?
Lunsumio targets CD20, which, while present on follicular lymphoma cells, is also present on some normal B cells. As a result, Lunsumio can also kill normal B cells, increasing the risk of serious infections. Other common side effects of Lunsumio include fever, cytokine release syndrome, fatigue, rash, fever, headache, low white blood cell count, low red blood cell count, low platelet count, and increased glucose and uric acid levels in the blood. Some side effects, such as cytokine release syndrome, neurological toxicities, or infections, can become serious or life-threatening. Patients and caregivers receive careful instructions to monitor for signs and symptoms of side effects. These conditions are managed by the healthcare provider.
Cytokine release syndrome (CRS)
CRS is caused by a widespread release of molecules called cytokines, which can cause inflammation and can affect the function of various organs. Cytokines may be released by the T cells to which Lunsumio binds, or by other immune cells in the patient’s body. Signs and symptoms of CRS include fever, headache, nausea, low blood pressure, and weakness. CRS typically occurs within 3 days after the most recent dose of Lunsumio. A healthcare provider should be immediately notified if symptoms occur.
Neurological toxicities
Some of the cytokines released during CRS can result in disruption of the blood–brain barrier, leading to the development of neurological toxicities. Symptoms of neurological toxicities include headache, tremors, seizures, loss of consciousness, confusion, difficulty with speech, and loss of balance.
Tumor flare
Lunsumio may cause worsening of the follicular lymphoma and worsening of cancer-related problems.
For a more complete list of possible side effects, see the full prescribing information.
Manufacturer
Genentech
Approval
FDA and EMA
Links to drug websites
Last updated: February 3, 2023
How is this drug name pronounced?
Mosunetuzumab: moh-SUN-eh-TOO-zoo-mab
Lunsumio: lun-SUM-ee-oh
What cancer(s) does this drug treat?
Follicular lymphoma
Lunsumio is approved for:
- Patients with follicular lymphoma who have received at least two prior treatments for their disease, but whose cancer either did not respond to treatment or has since come back.
Limitations of use
Limitations: Lunsumio should only be administered in a treatment center and by adequately trained healthcare professionals who can ensure proper monitoring and immediate management of side effects associated with the drug.
Age: The safety and efficacy of Lunsumio in patients under 18 years of age have not been established.
Pregnancy/Breastfeeding: Lunsumio is not recommended for use during pregnancy due to potential for harm to the fetus. Patients are advised to use contraception during treatment with Lunsumio and for at least 3 months after the last dose of Lunsumio. The risks associated with Lunsumio during breastfeeding are not known and cannot be ruled out. Due to the potential for adverse reactions in the breastfed child, patients are advised not to breastfeed during treatment with Lunsumio and for at least 3 months after the last dose of Lunsumio.
Interactions with other drugs: Dose adjustments may become necessary for patients treated with CYP450 substrates (e.g., warfarin, cyclosporin, theophylline) at the start of treatment with Lunsumio.
Live virus vaccination: Vaccination with attenuated live virus vaccines (e.g., chickenpox, measles/mumps/rubella [MMR]) is not recommended during treatment with Lunsumio.
What type of immunotherapy is this?
How does this drug work?
Targets:
- CD20 on B cells
- CD3 receptor on T cells
Lunsumio is a “bispecific antibody”. Antibodies are molecules that can bind to a particular target molecule. Bispecific antibodies are artificially made in the laboratory and can bind to two different targets at the same time. Lunsumio binds to:
- a molecule called CD20 on the surface of follicular lymphoma cells and some healthy B cells. CD20 is present in much higher quantities on the surface of cancer cells than normal healthy cells.
- a molecule called CD3 on the surface of T cells – the primary immune cells involved in killing cancer cells. CD3 is part of the T cell receptor, which is critical to the function of the T cell and is involved in stimulating the T cell to become active.
Because Lunsumio can bind to one molecule on follicular lymphoma cells and one on T cells at the same time, it acts as a bridge and keeps the T cell in close contact with the cancer cell. By binding CD3 on the T cell, Lunsumio also stimulates the T cell to become activated and kill the cell it is bound to. Bispecific antibodies that direct T cells to kill cancer cells by binding to both cells at the same time are known as bispecific T cell engagers (BiTEs).

How is the drug given to the patient?
About 1 hour to 30 minutes prior to receiving each dose of Lunsumio in treatment cycle 1 and 2 and if needed in subsequent treatment cycles, patients receive several medications to help reduce the risk of cytokine release syndrome:
- A corticosteroid (dexamethasone or methylprednisolone)
- An antihistamine (diphenhydramine)
- A painkiller and fever reducer (paracetamol)
Patients should be well hydrated before they receive Lunsumio, which is administered through a tube in the vein (intravenous (IV) infusion). During the first treatment cycle (a 21-day period), each dose of Lunsumio is delivered over the course of at least four hours. A low dose of Lunsumio is administered on day 1, a second, slightly higher dose of Lunsumio is given on day 8, and the first full dose of Lunsumio is administered on day 15 of treatment cycle 1. During the second treatment cycle, a full dose of Lunsumio is administered on day 1 over the course of 2 hours. For every subsequent treatment cycle, half of the full dose of Lunsumio is given on day 1 over the course of 2 hours. Before and periodically during the treatment, blood tests are performed to monitor for side effects.

What are the observed clinical results?
It is important to keep in mind that each patient’s outcome is individual and may be different from the results found in the clinical studies. In addition, immunotherapy can sometimes take several months to yield an observable treatment response.
Follicular lymphoma
In a clinical study, 90 patients with follicular lymphoma who had received at least two prior treatments for their disease, but whose cancer either did not respond to treatment or had since come back were treated with Lunsumio. At a median follow-up of 25 months:

What are the potential side effects?
Lunsumio targets CD20, which, while present on follicular lymphoma cells, is also present on some normal B cells. As a result, Lunsumio can also kill normal B cells, increasing the risk of serious infections. Other common side effects of Lunsumio include fever, cytokine release syndrome, fatigue, rash, fever, headache, low white blood cell count, low red blood cell count, low platelet count, increased glucose and uric acid levels in the blood. Some side effects, such as cytokine release syndrome, neurological toxicities, or infections, can become serious or life-threatening. Patients and caregivers receive careful instructions to monitor for signs and symptoms of side effects. These conditions are managed by the healthcare provider.
Cytokine release syndrome (CRS)
CRS is caused by a widespread release of molecules called cytokines, which can cause inflammation and can affect the function of various organs. Cytokines may be released by the T cells to which Lunsumio binds, or by other immune cells in the patient’s body. Signs and symptoms of CRS include fever, headache, nausea, low blood pressure, and weakness. CRS typically occurs within 3 days after the most recent dose of Lunsumio during treatment cycle 1. A healthcare provider should be immediately notified if symptoms occur.
Neurological toxicities
Some of the cytokines released during CRS can result in disruption of the blood–brain barrier, leading to the development of neurological toxicities. Symptoms of neurological toxicities include headache, tremors, seizures, loss of consciousness, confusion, difficulty with speech, and loss of balance.
Tumor flare
Lunsumio may cause worsening of the follicular lymphoma and worsening of cancer-related problems.
For a more complete list of possible side effects, see the full prescribing information.
Manufacturer
Roche
Approval
FDA and EMA
Links to drug websites
Last updated: February 3, 2023






