How is this drug name pronounced?
Nivolumab plus hyaluronidase: nih-VOL-yoo-mab plus HY-al-yoo-RAH-nih-days
Opdivo Qvantig: op-DEE-voh cue-VAN-tig
What cancer(s) does this drug treat?
Opdivo Qvantig is approved for:
Kidney cancer (renal cell carcinoma)
Melanoma
Lung cancer
Head and neck squamous cell cancer
Bladder and urinary tract (urothelial cell) cancer
Colorectal cancer (MSI-H/dMMR)
Liver cancer
Esophageal cancer
Stomach cancer
Advanced kidney cancer (Renal cell carcinoma)
Opdivo Qvantig is approved for:
- Adult patients with intermediate- or poor-risk advanced renal cell carcinoma (kidney cancer) who have previously been treated with Opdivo in combination with ipilimumab (Yervoy). Opdivo Qvantig cannot be used in combination with ipilimumab (Yervoy) in these patients.
- Adult patients with advanced renal cell carcinoma. In such cases, Opdivo Qvantig may be used in combination with cabozantinib (e.g., Cabometyx).
- Adult patients with advanced renal cell carcinoma who have previously been treated with an anti-angiogenic treatment. In such cases, Opdivo Qvantig is used on its own.
Advanced melanoma
Opdivo Qvantig is approved for:
- Adult patients with advanced melanoma that is metastatic (cancer has spread to other parts of the body from the original cancer site) or cannot be completely removed by surgery. Opdivo Qvantig may be used after combination treatment with Opdivo and ipilimumab (Yervoy), but cannot be used in combination with Yervoy.
- Adult patients with melanoma (Stage IIB, IIC, III, or IV) that, together with lymph nodes or other tissue the cancer has spread to (metastases), was completely removed by surgery. In such cases, Opdivo Qvantig is used to help keep melanoma from coming back.
Advanced lung cancer
Opdivo Qvantig is approved for:
- Adult patients with non-small cell lung cancer that can be removed by surgery. In such cases, Opdivo Qvantig is used in combination with platinum-containing therapy prior to surgery, in an effort to reduce the size of the tumor.
- Adult patients with non-small cell lung cancer that can be removed by surgery and do not have abnormal EGFR or ALK genes. In such cases, Opdivo Qvantig is used in combination with platinum-containing therapy prior to surgery, in an effort to reduce the size of the tumor, and then is continued after surgery, to reduce the risk of the tumor coming back.
- Adult patients with non-small cell lung cancer that has spread to other parts of the body and has progressed on or after treatment with a platinum-containing chemotherapy. If the patient’s tumor has an abnormal EGFR or ALK gene, the patient has to have also tried an FDA-approved therapy for tumors with such abnormal genes prior to receiving Opdivo Qvantig. Opdivo Qvantig cannot be used in combination with ipilimumab (Yervoy) in these patients.
Head and neck squamous cell cancer
Opdivo Qvantig is approved for:
- Adult patients with head and neck squamous cell carcinoma whose cancer has come back, spread, or progressed (gotten worse) on or after treatment with chemotherapy that contains platinum. In such cases, Opdivo Qvantig is used on its own.
Advanced bladder and urinary tract (urothelial cell) cancer
Opdivo Qvantig is approved for:
- Adult patients with urothelial carcinoma who are at high risk of their cancer coming back after surgical removal of all known disease.
- Adult patients with urothelial carcinoma that cannot be removed by surgery or has spread to other parts of the body, and who have not yet received treatment for their advanced disease. In such cases, Opdivo Qvantig is used in combination with cisplatin and gemcitabine chemotherapy.
- Adult patients with advanced urothelial carcinoma that is locally advanced or has spread to other parts of the body and that either progressed on or after treatment with a chemotherapy containing platinum, or came back within 12 months of surgery that was accompanied by prior or follow-up treatment with a chemotherapy containing platinum.
Advanced colorectal cancer (MSI-H/dMMR)
Opdivo Qvantig is approved for:
- Adult patients with colon or rectal cancer that has spread and is microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR), and who have been treated with a fluoropyrimidine, oxaliplatin, and irinotecan chemotherapy (alone or after treatment with Opdivo and ipilimumab (Yervoy)), and the treatment did not work or stopped working. In such cases, patients are treated with Opdivo Qvantig. Opdivo Qvantig cannot be used in combination with ipilimumab (Yervoy) in these patients.
Advanced liver cancer
Opdivo Qvantig is approved for:
- Adult patients with hepatocellular carcinoma (liver cancer) who have been treated with Opdivo and ipilimumab (Yervoy) followed by sorafenib (Nexavar), and the treatment either did not work or stopped working In such cases, patients are treated with Opdivo Qvantig. Opdivo Qvantig cannot be used in combination with ipilimumab (Yervoy) in these patients.
Esophageal cancer
Opdivo Qvantig is approved for:
- Adult patients with esophageal cancer or gastroesophageal junction cancer that has been completely removed by surgery after being treated by chemotherapy and concurrent radiation, but cancer cells remain in the body after treatment.
- Adult patients with esophageal squamous cell carcinoma that tests positive for the PD-L1 molecule, is advanced and cannot be removed by surgery or has spread to other parts of the body, and who have not yet received treatment for their advanced disease. In such cases, Opdivo Qvantig is used in combination with a fluoropyrimidine- and platinum-containing chemotherapy. Opdivo Qvantig cannot be used in combination with ipilimumab (Yervoy) in these patients.
- Patients with esophageal squamous cell carcinoma that is advanced and cannot be removed by surgery, has come back, or has spread to other parts of the body, and who have been previously treated with fluoropyrimidine- and platinum-containing chemotherapy. In such cases, Opdivo Qvantig is used by itself.
- Adult patients with esophageal adenocarcinoma, gastroesophageal junction cancer, or gastric (stomach) cancer that has grown or spread to other parts of the body and tests positive for the PD-L1 molecule. In such cases, Opdivo Qvantig is used in combination with fluoropyrimidine and platinum-containing chemotherapy.
Advanced stomach cancer
Opdivo Qvantig is approved for:
- Adult patients with stomach (gastric) cancer, gastroesophageal junction or esophageal adenocarcinoma that has grown or spread to other parts of the body and tests positive for the PD-L1 molecule. In such cases, Opdivo Qvantig is used in combination with fluoropyrimidine- and platinum-containing chemotherapy.
Limitations of use
Age: The safety and efficacy of Opdivo Qvantig in patients under 18 years of age have not been established.
Pregnancy/Breastfeeding: Opdivo Qvantig can cause harm to a fetus, and is not recommended for use during pregnancy. Women are advised to use contraception during treatment, and for at least 5 months after the last dose of Opdivo Qvantig. The risks associated with Opdivo Qvantig during breastfeeding are not known and cannot be ruled out; due to the potential for serious adverse reactions in the breastfed child, women are advised not to breastfeed during treatment and for 5 months after the last dose of Opdivo Qvantig.
Complications of stem cell transplant: Serious and life-threatening complications that can lead to death can occur in patients who have received a stem cell transplant from a stem cell donor before or after being treated with Opdivo Qvantig. The benefit of treatment with Opdivo Qvantig versus the possible risk of transplant-related complications (especially in patients with a history of graft-versus-host disease) should be carefully considered.
What type of immunotherapy is this?
- PD-1 blockade
How does this drug work?
- Target: PD-1
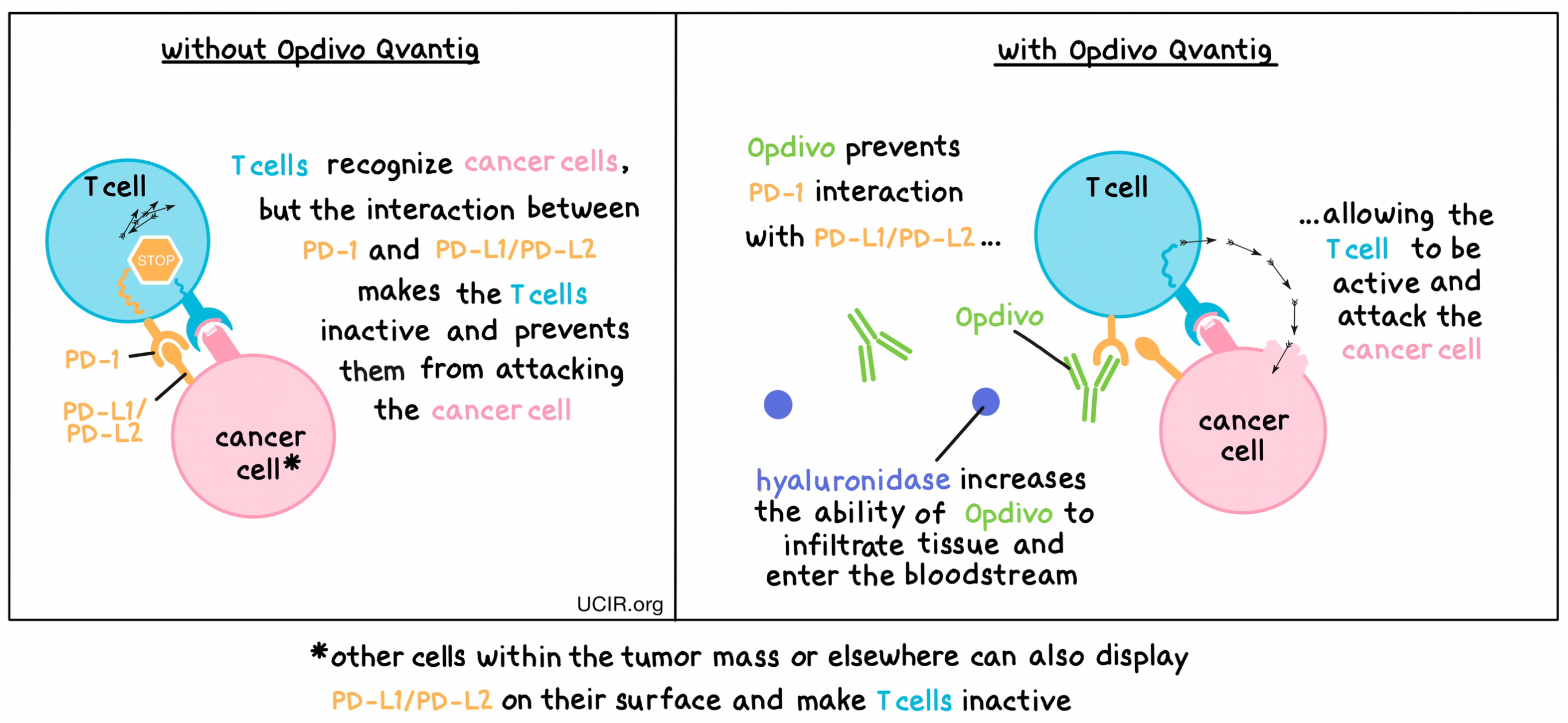
Opdivo Qvantig is a combination treatment of nivolumab (Opdivo) and hyaluronidase. Opdivo is the therapeutic component of the treatment, while hyaluronidase is added to increase the ability for Opdivo to infiltrate into tissue under the skin and enter the bloodstream.
Opdivo is an antibody that attaches to a molecule called PD-1, which is present on the surface of T cells – the primary immune cells involved in killing cancer cells. In healthy tissues, PD-1 acts as a brake that keeps T cells from creating an immune reaction that gets out of control. However, cancers can hijack this safety mechanism and prevent T cells from doing their job – killing the cancer cells. This can happen when cancer cells or other cells within the tumor mass display on their surface molecules called PD-L1 or PD-L2. When PD-L1 or PD-L2 interact with PD-1 on T cells, the T cells become inactive and do not attack the cancer cells. Opdivo binds to the PD-1 molecules on T cells in such a way that it prevents the interaction between PD-1 and PD-L1/PD-L2, and allows the T cells to be active and attack the cancer cells.
How is this drug given to the patient?
Opdivo Qvantig is administered via an injection under the skin (subcutaneous injection) in the abdomen or thigh over approximately 3-5 minutes every 2, 3, or 4 weeks, depending on the cancer type and treatment regimen. It must be given by a healthcare professional, but does not require a hospital stay.
What are the observed clinical results?
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies described below. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Opdivo Qvantig (subcutaneous administration) vs. Opdivo (intravenous administration)
In a clinical trial, 495 patients with renal cell carcinoma that was advanced or had spread to other parts of the body were treated with either Opdivo Qvantig, administered as an injection subcutaneously (under the skin) in the thigh or abdomen every 4 weeks or Opdivo, administered intravenously (though a tube in the vein) every 2 weeks. In both settings, patients were found to be exposed to similar levels of the active drug nivolumab and patients had similar rates of response. Based on these results, Opdivo Qvantig was approved as an alternative to Opdivo, and the effectiveness of Opdivo Qvantig for the above and below listed indications has been proven in clinical routine care. The efficacy of Opdivo has been established in clinical trials for:
Advanced kidney cancer
Advanced melanoma (metastatic or not removable by surgery)
Advanced melanoma (completely removed by surgery)
Advanced non-small cell lung cancer (squamous and non-squamous)
Head and neck squamous cell cancer
Advanced bladder and urinary tract cancer
Advanced colorectal cancer (MSI-H/dMMR)
Advanced liver cancer
Advanced esophageal cancer
Advanced stomach cancer
Advanced kidney cancer
In a clinical study, 847 patients with previously untreated advanced kidney cancer were treated with either a combination of Opdivo and ipilimumab (Yervoy) or with sunitinib (an angiogenesis inhibitor):
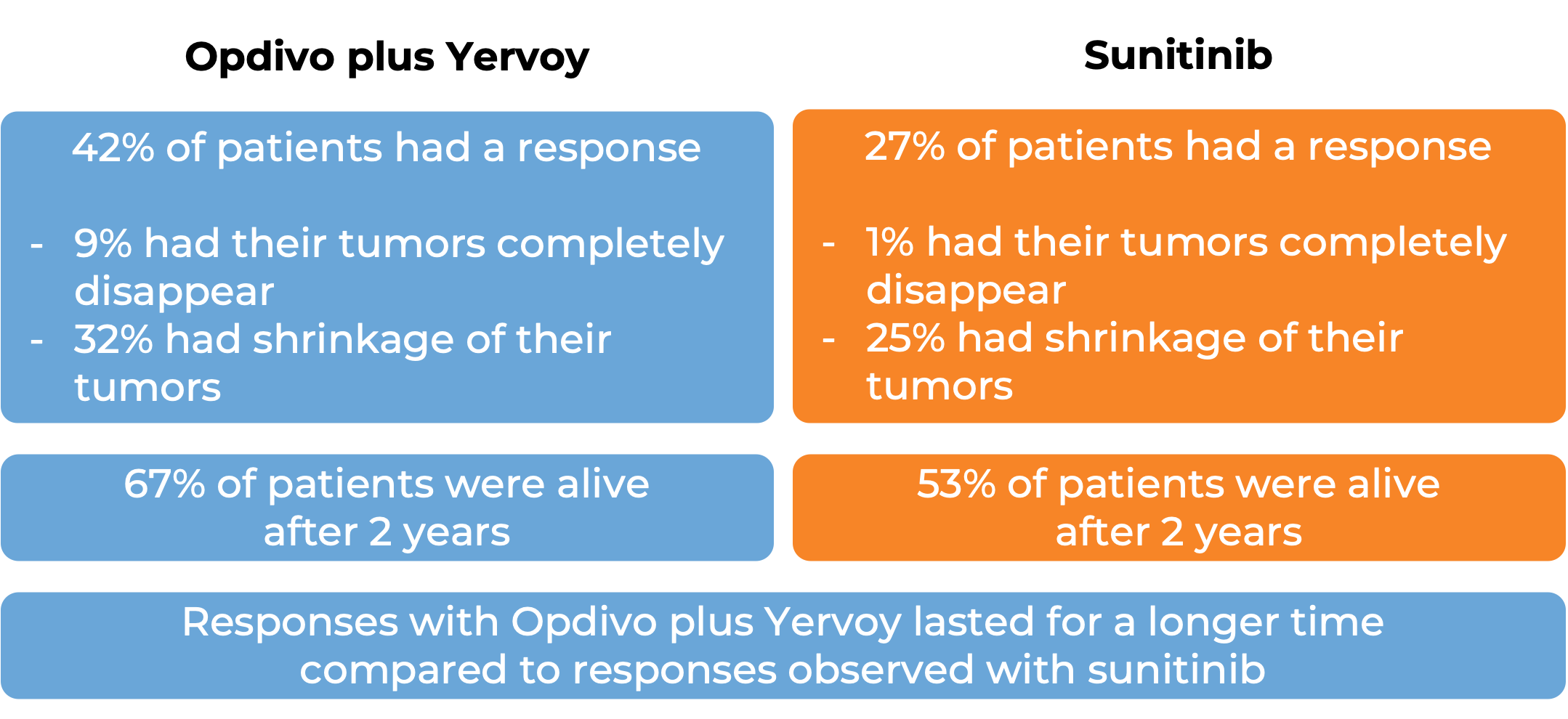
Note: Rounding percentages after the decimal can cause inconsistencies between the total and the sum of the parts.
In another clinical study, 651 patients with advanced renal cell carcinoma who had not received any other treatment for their advanced disease, were treated with either Opdivo and cabozantinib or sunitinib (an angiogenesis inhibitor). At at a median follow-up of 18 months (and extended median follow-up of 44 months for survival):
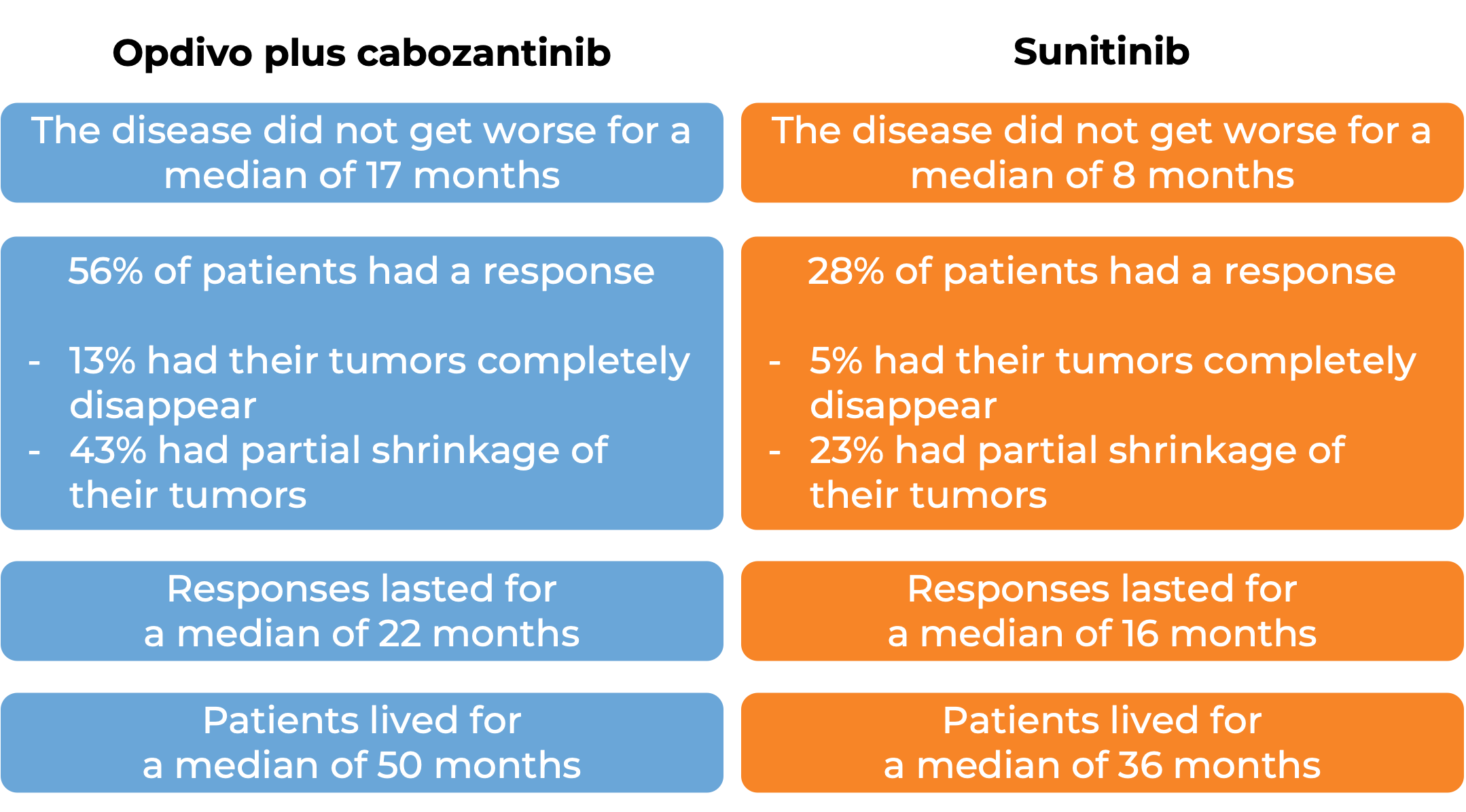
In another clinical study, 821 patients with previously treated advanced kidney cancer were treated with either Opdivo or everolimus (an oral chemotherapy):
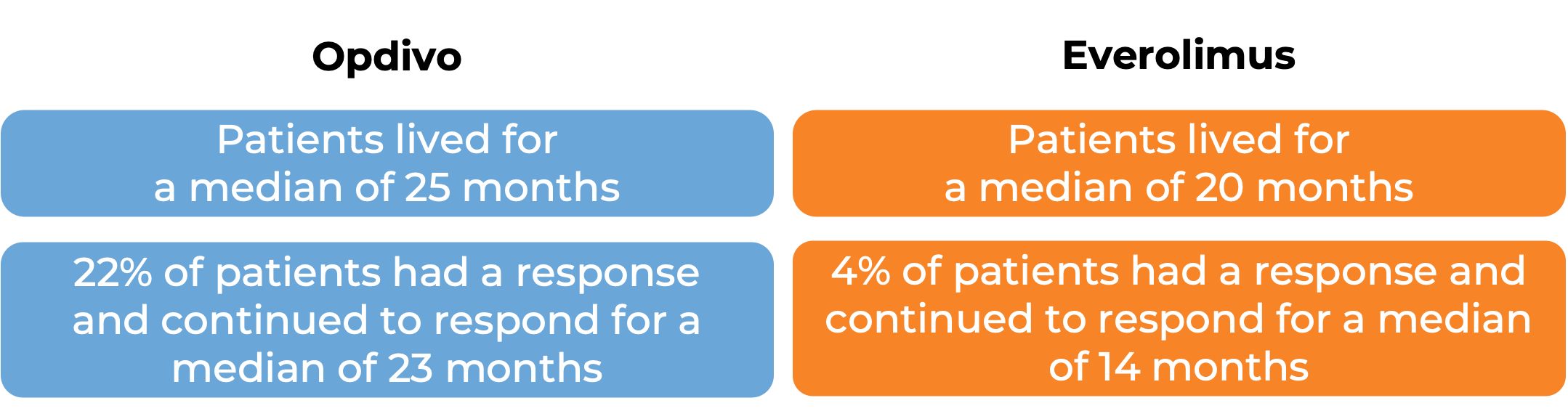
(For a definition of median click HERE.)
Advanced melanoma (metastatic or not removable by surgery)
In a clinical trial, 405 patients with melanoma that had spread to other parts of the body or could not be removed by surgery and whose disease had gotten worse or come back during or after treatment with ipilimumab (Yervoy), were treated with Opdivo or chemotherapy (dacarbazine or carboplatin plus paclitaxel).

(For the definition of “median” click HERE.)
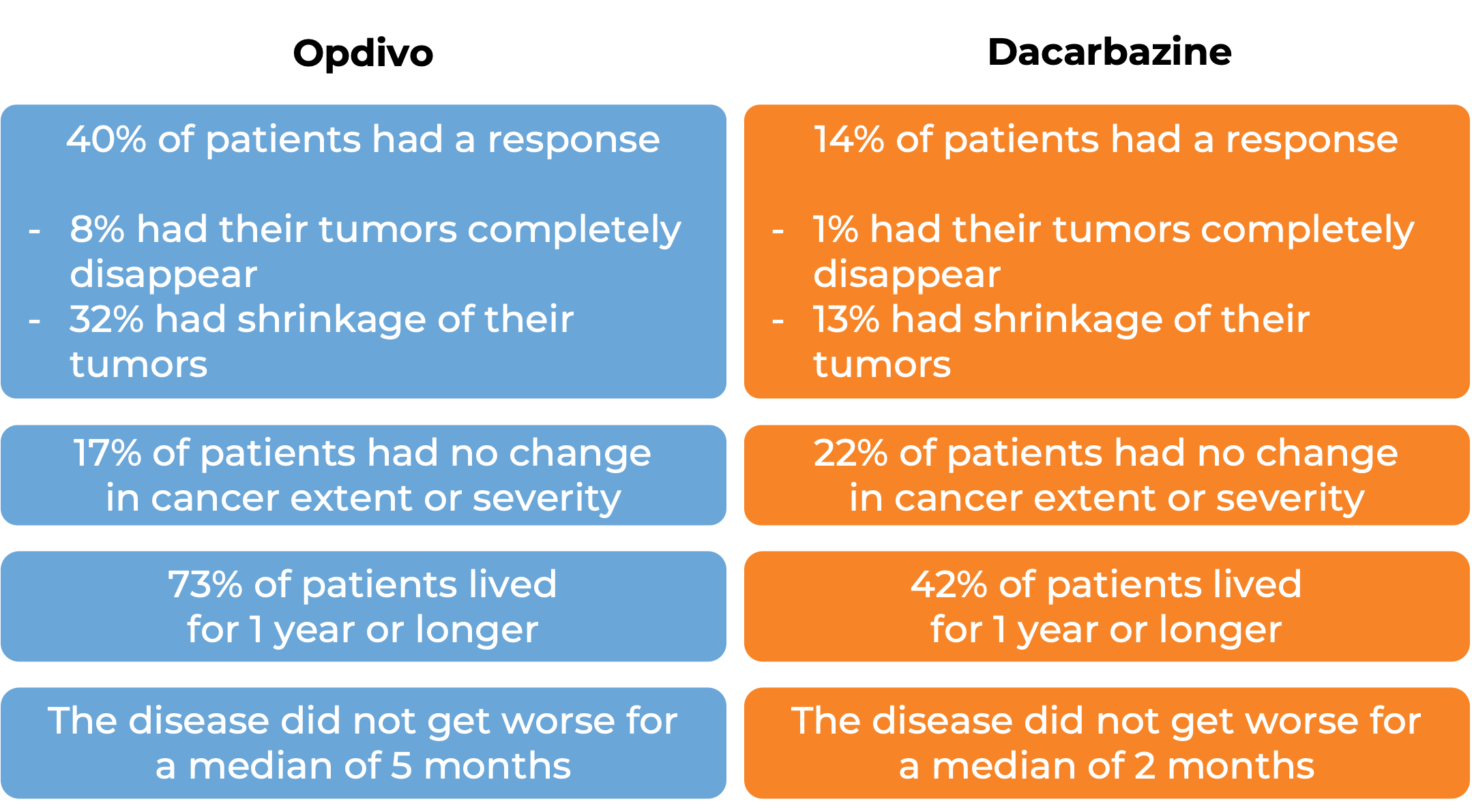
In another clinical trial, 418 patients with melanoma that had spread to other parts of the body or could not be removed by surgery were treated with Opdivo or dacarbazine. At a median follow-up of 17 months:
In another clinical trial, 945 patients with melanoma that had spread to other parts of the body or could not be removed by surgery, and who had not received treatment for their advanced disease were treated with Opdivo plus ipilimumab (Yervoy), Opdivo alone, or Yervoy alone.
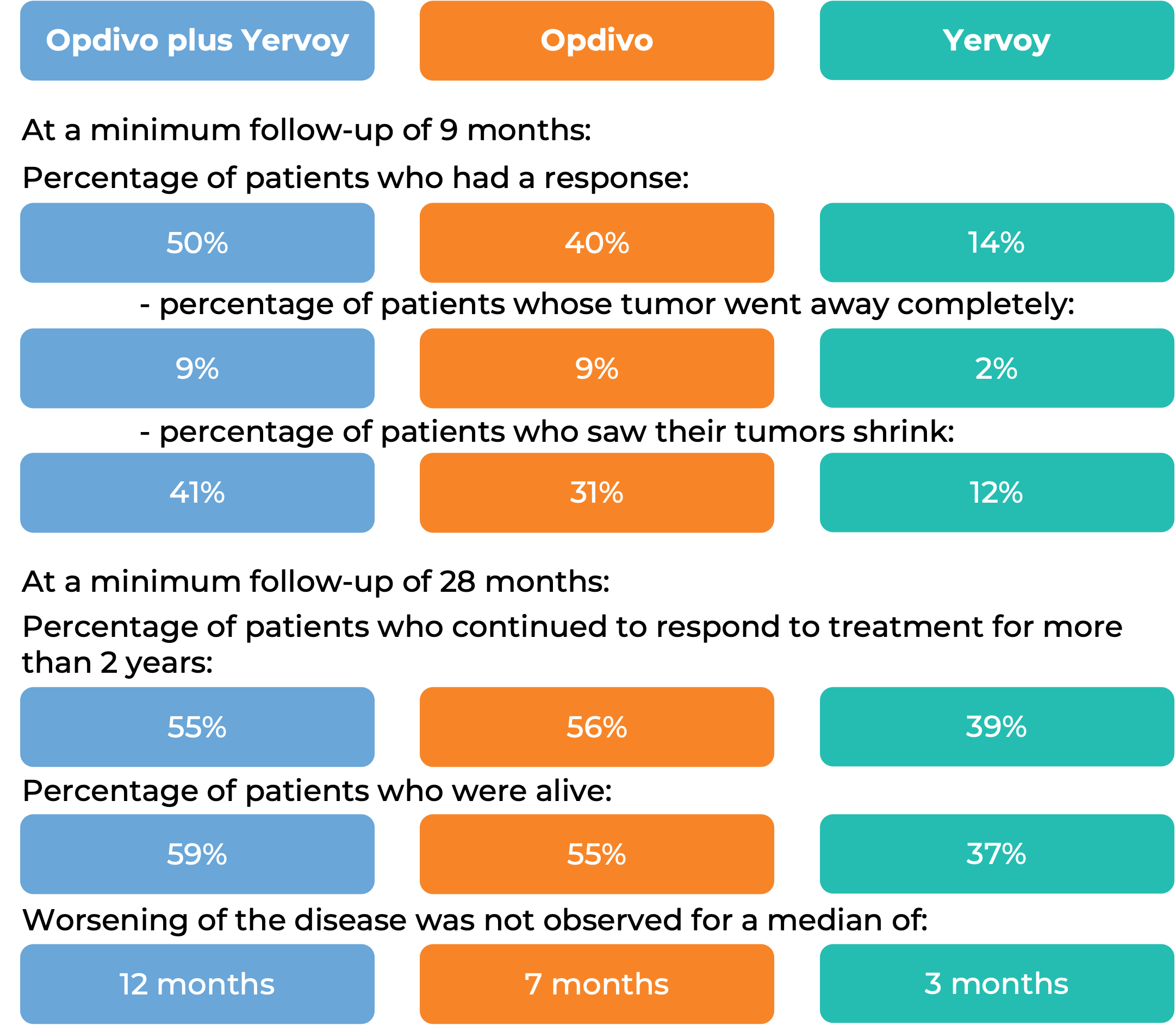
(For a definition of median click HERE.)
Advanced melanoma (completely removed by surgery)
In a clinical trial, 790 patients with Stage IIB/C melanoma that was completely removed by surgery were treated with either Opdivo or a placebo to prevent the cancer from coming back.

In a clinical study, 906 patients with Stage IIIB/C or IV melanoma that was completely removed by surgery were treated with either Opdivo or ipilimumab (Yervoy) to prevent the cancer from coming back. At a minimum follow-up of 62 months:
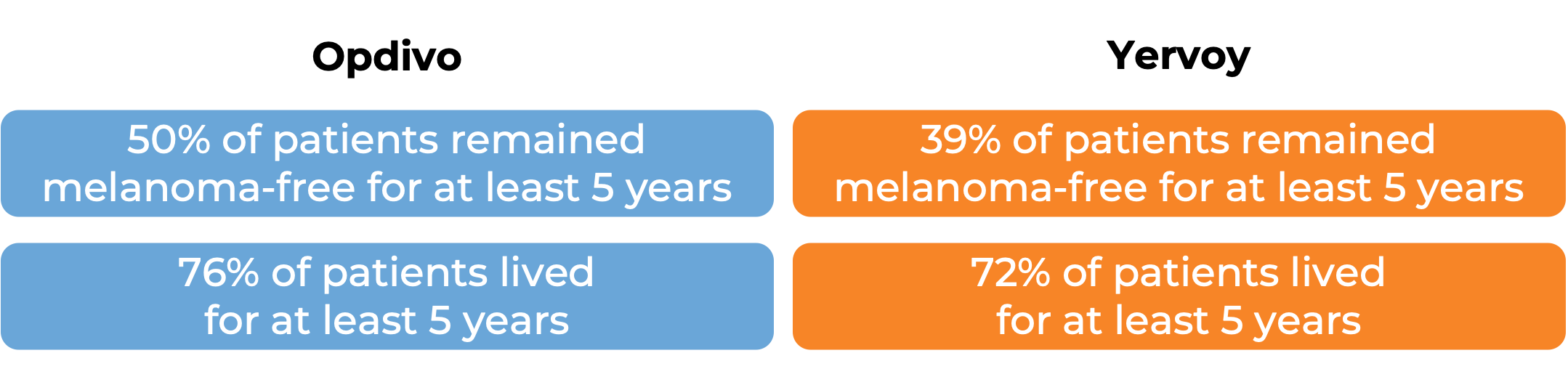
(For the definition of “median” click HERE.)
Advanced non-small cell lung cancer (squamous and non-squamous)
In a clinical study, 358 patients with non-small cell lung cancer that could be removed by surgery were treated with either Opdivo in combination with platinum-containing chemotherapy, or with platinum-containing chemotherapy alone prior to surgery to remove their cancer. At a minimum follow-up of 21 months:
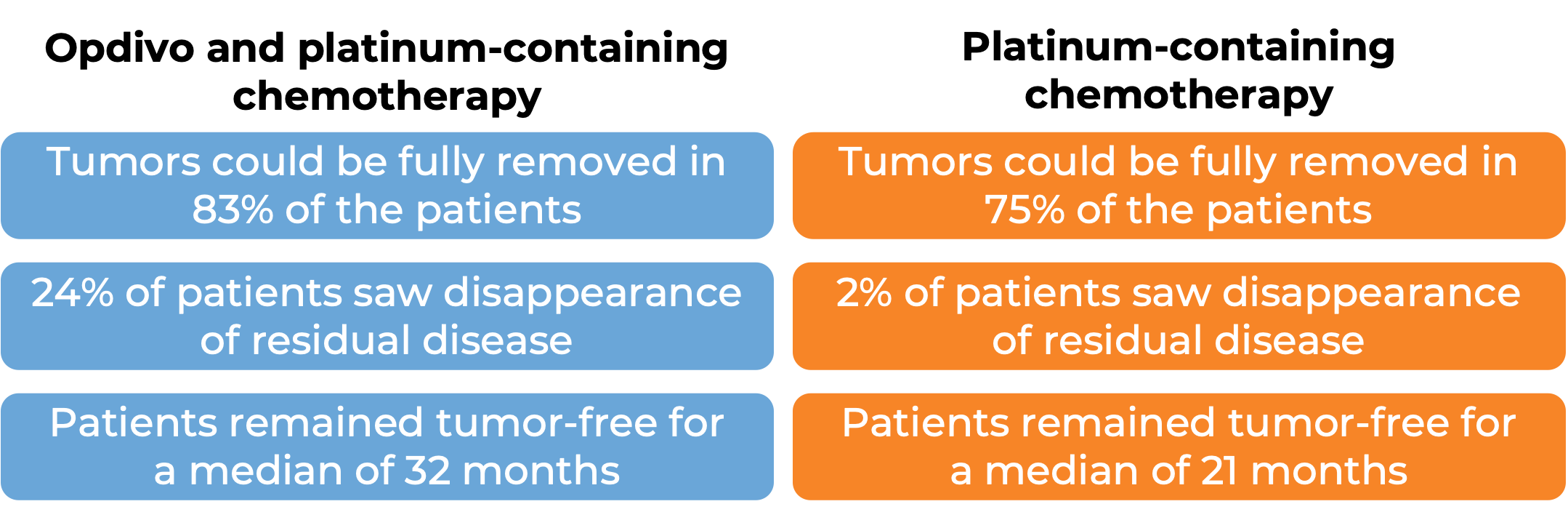
(For the definition of “median” click HERE.)
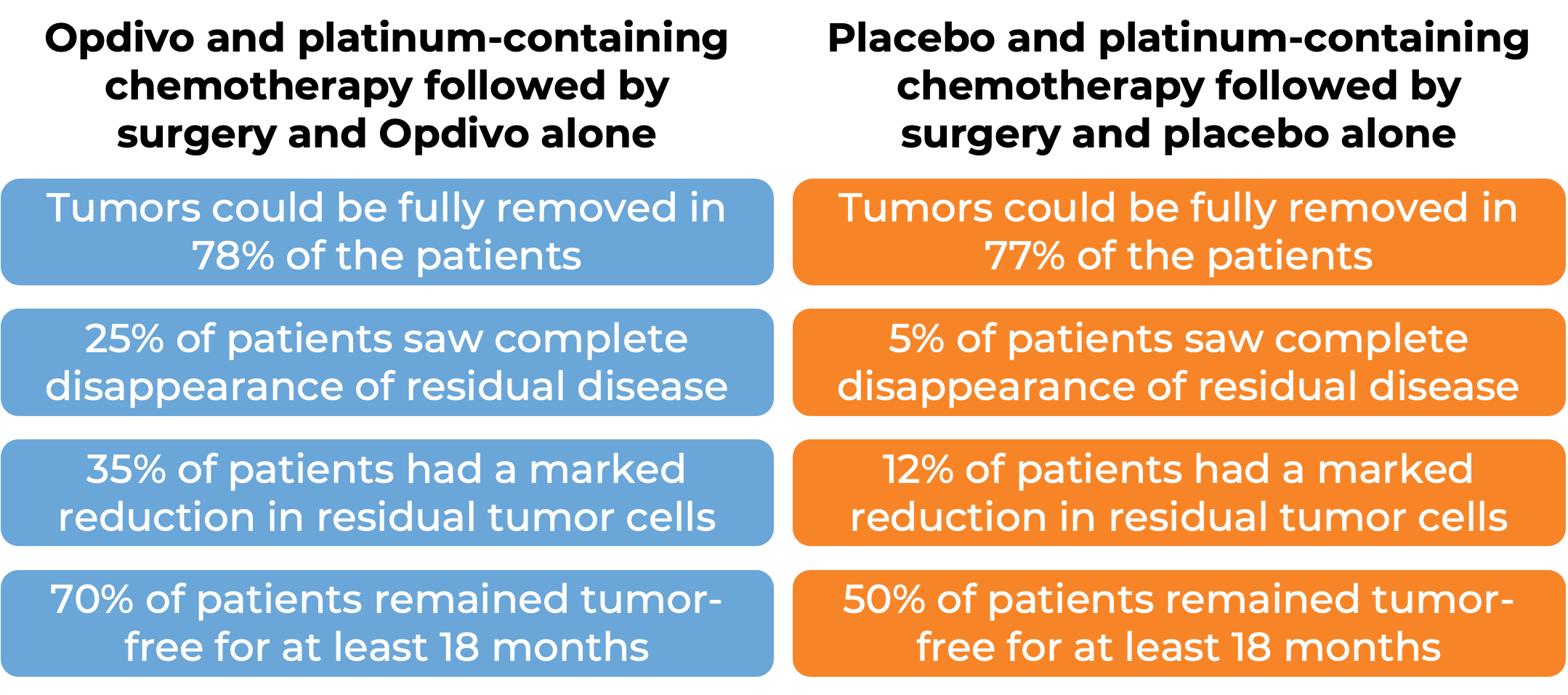
In a clinical trial, 461 patients with non-small cell lung cancer that could be removed by surgery were treated with either:
- Opdivo in combination with platinum-containing chemotherapy prior to surgery to remove their cancer, and Opdivo after surgery, OR
- Placebo and platinum-containing chemotherapy prior to surgery to remove their cancer, and placebo after surgery.
At a median follow-up of 25 months:
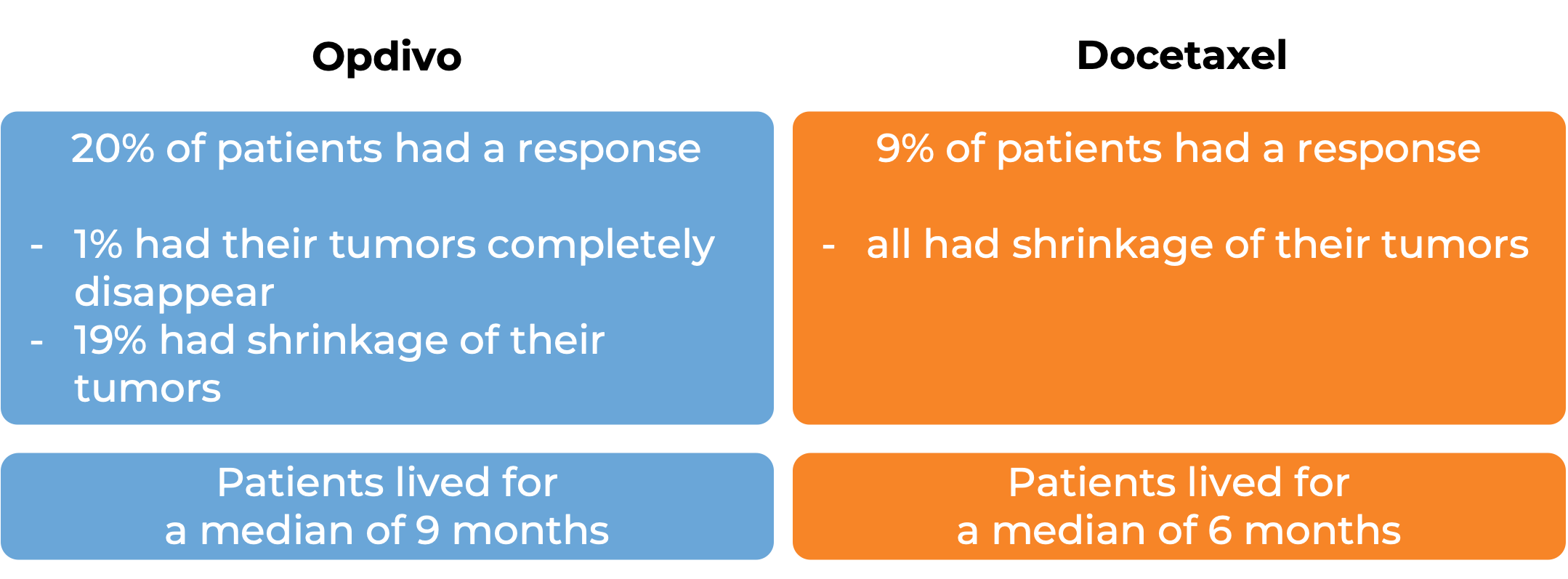
In a clinical study, 272 patients with previously treated squamous non-small cell lung cancer that had spread to other parts of the body were treated with either Opdivo or docetaxel (a chemotherapy):
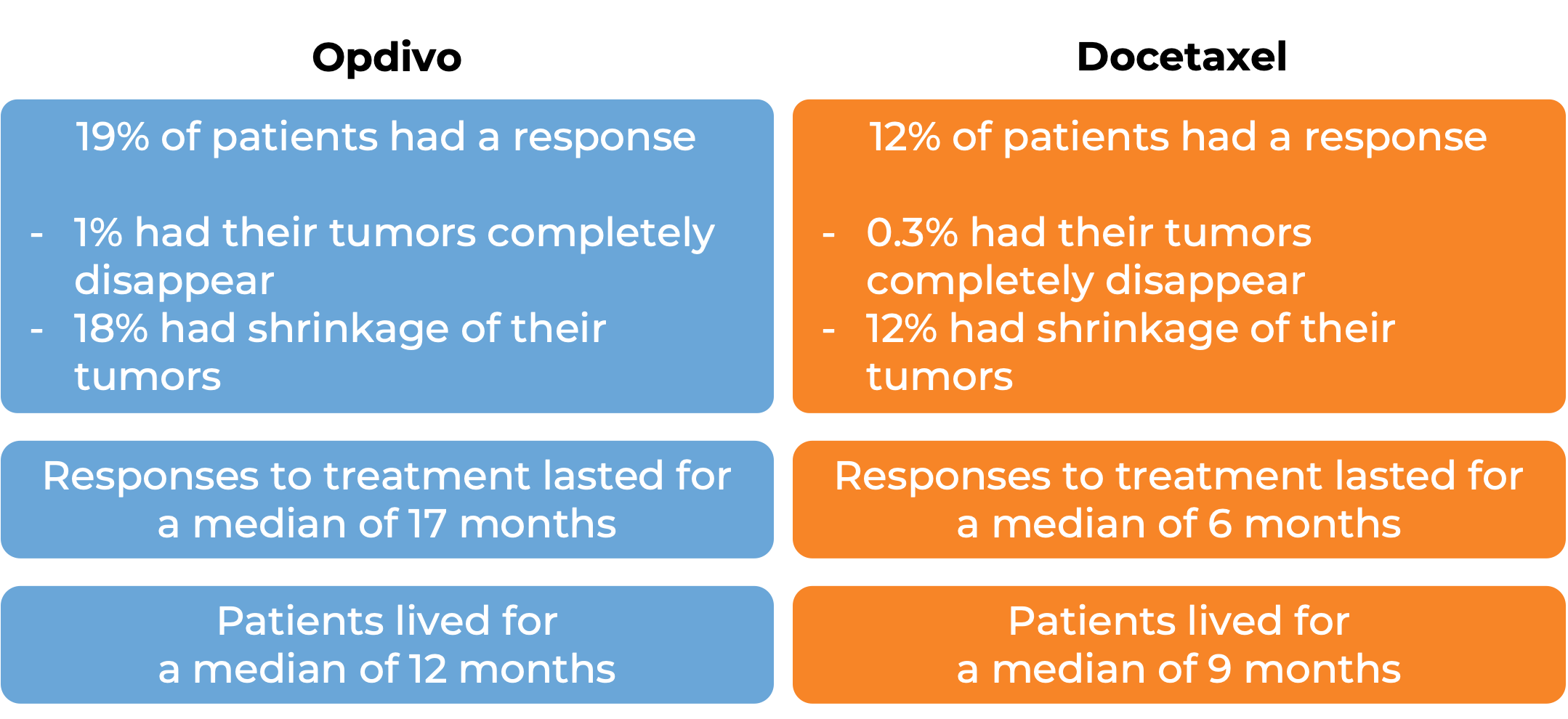
In a clinical study, 582 patients with previously treated non-squamous non-small cell lung cancer that had grown or spread to other parts of the body were treated with either Opdivo or docetaxel (a chemotherapy):
Head and neck squamous cell cancer
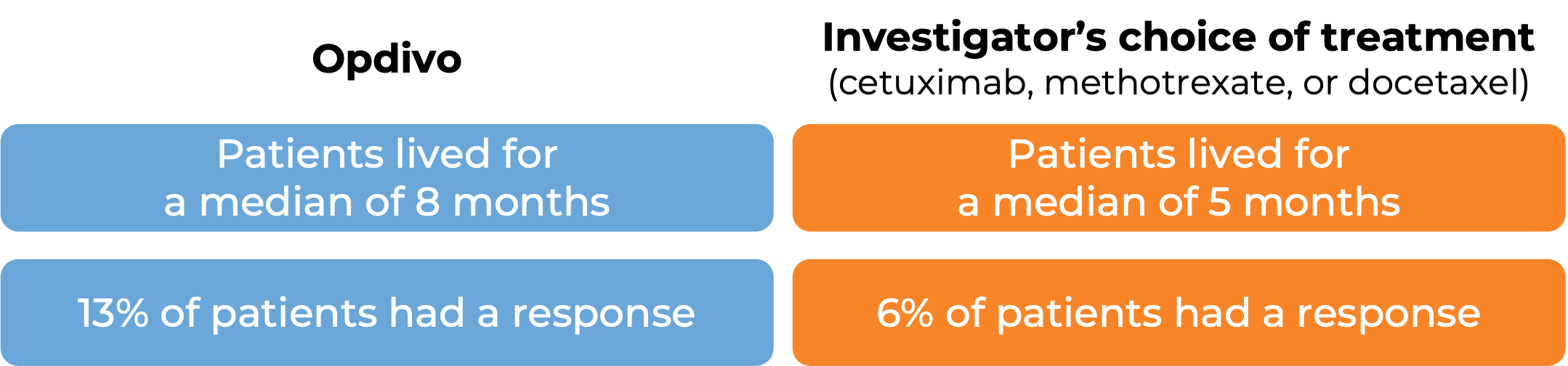
In a clinical study, 361 patients with head and neck squamous cell cancer were treated with Opdivo or with the investigator’s choice of treatment (cetuximab, methotrexate, or docetaxel).
(For a definition of median click HERE.)
Advanced bladder and urinary tract cancer
In a clinical study, 709 patients with advanced bladder cancer whose cancer had been removed by surgery, but who were at a high risk of coming back, were treated with Opdivo or placebo. At a median follow-up of 20 to 21 months:
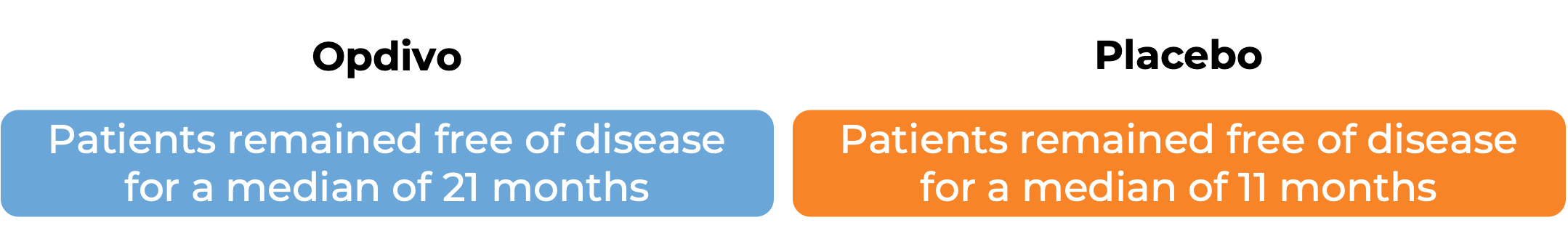
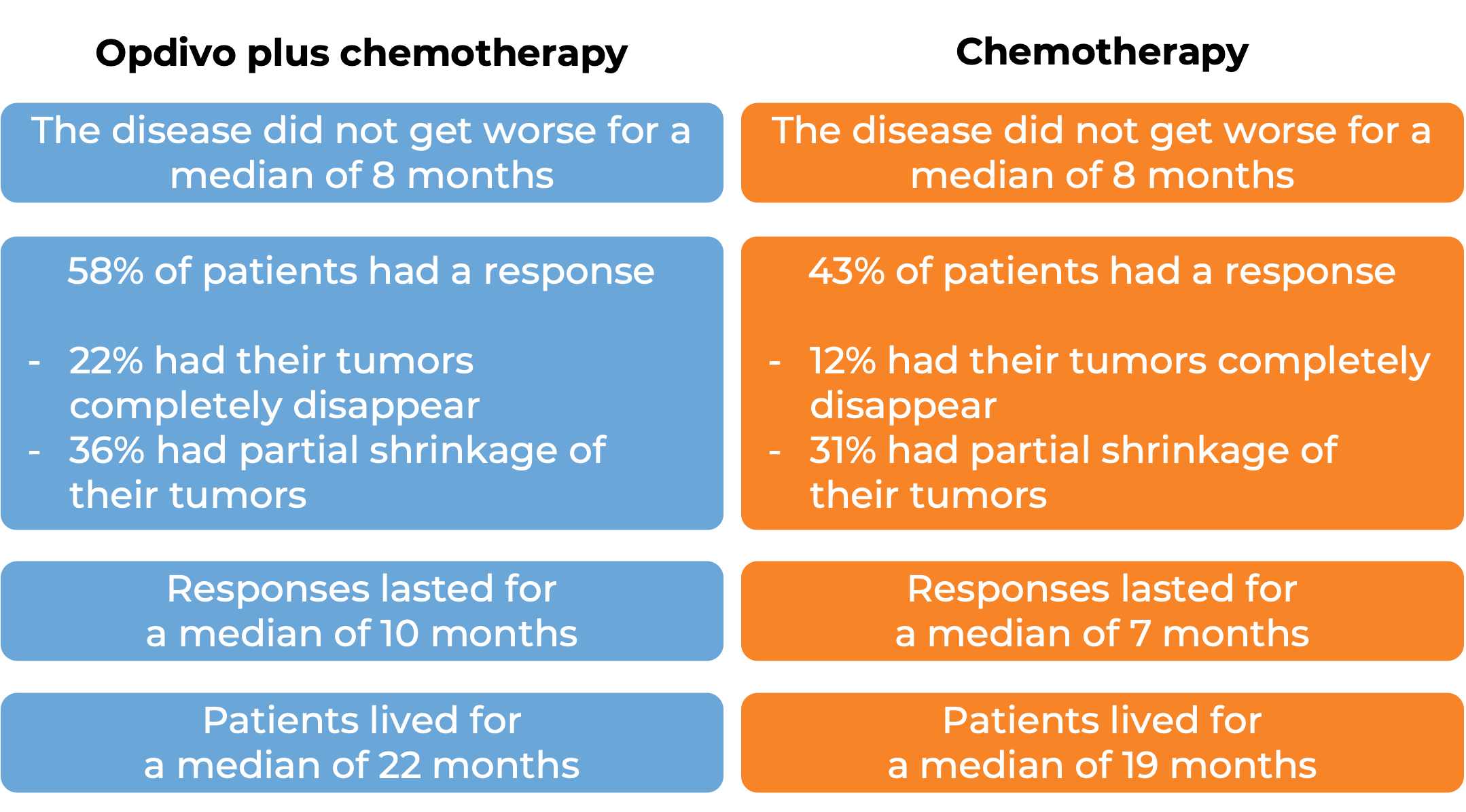
In another clinical study, 608 patients with urothelial carcinoma (the most common type of bladder and urinary tract cancer) that could not be removed by surgery or had spread to other parts of the body, and who had not received treatment for their advanced disease, were treated with Opdivo plus cisplatin and gemcitabine chemotherapy or chemotherapy alone. At a median follow-up of 34 months:
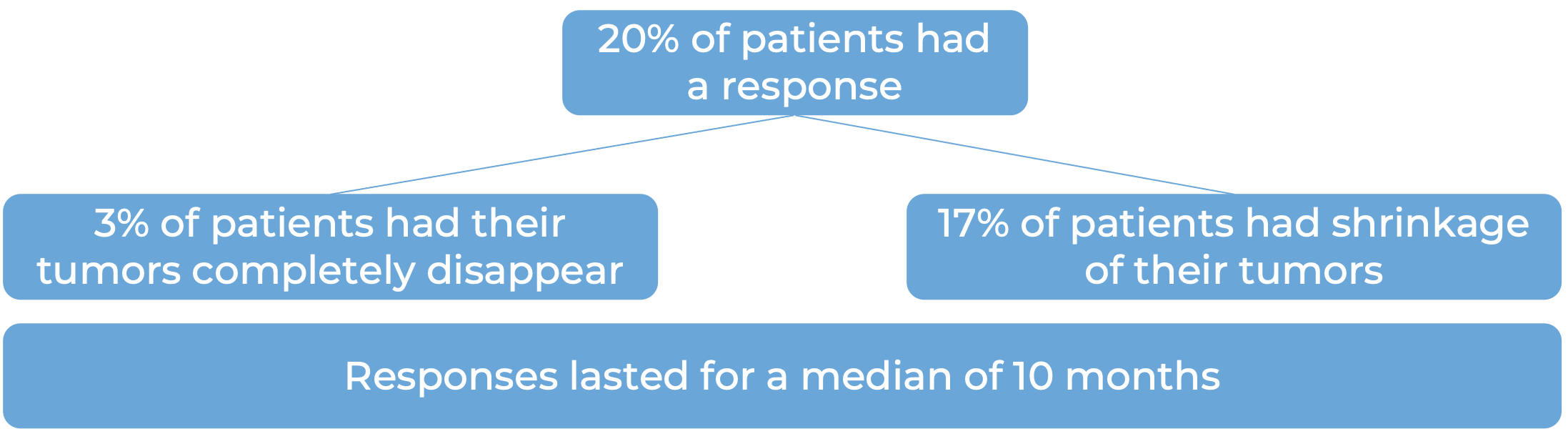
In another clinical study, 270 patients with advanced urothelial carcinoma (the most common type of bladder and urinary tract cancer) that had grown or spread, who had been treated with chemotherapy containing platinum, and it did not work or stopped working, were treated with Opdivo:
Advanced colorectal cancer (MSI-H/dMMR)
In a clinical study, 193 patients with microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) colon or rectal (“colorectal”) cancer (including patients who have been treated with a fluoropyrimidine, oxaliplatin, and irinotecan chemotherapy, and the treatment did not work or stopped working) were treated with Opdivo or a combination of Opdivo and ipilimumab (Yervoy).
At a median follow-up of 34 months among patients treated with Opdivo:
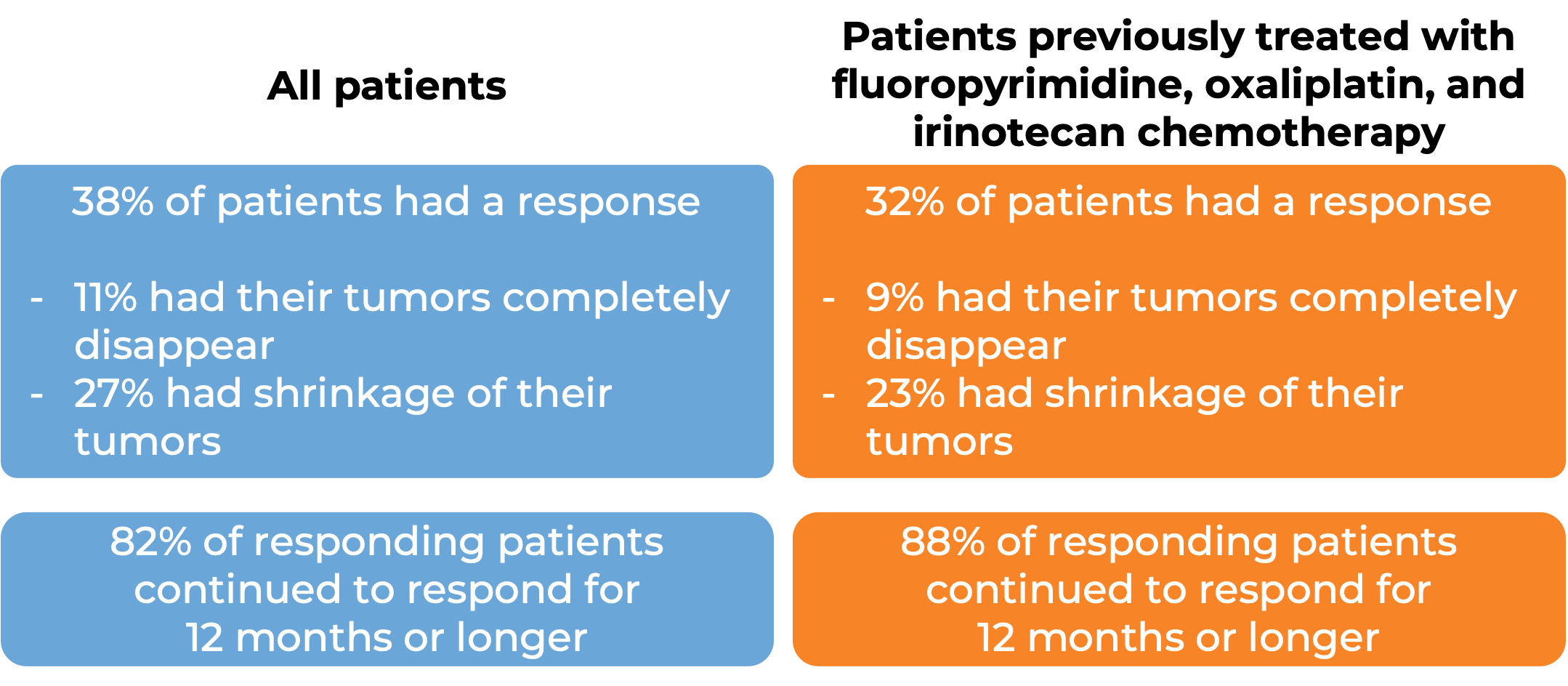
At a median follow-up of 28 months, among patients treated with Opdivo and ipilimumab (Yervoy):
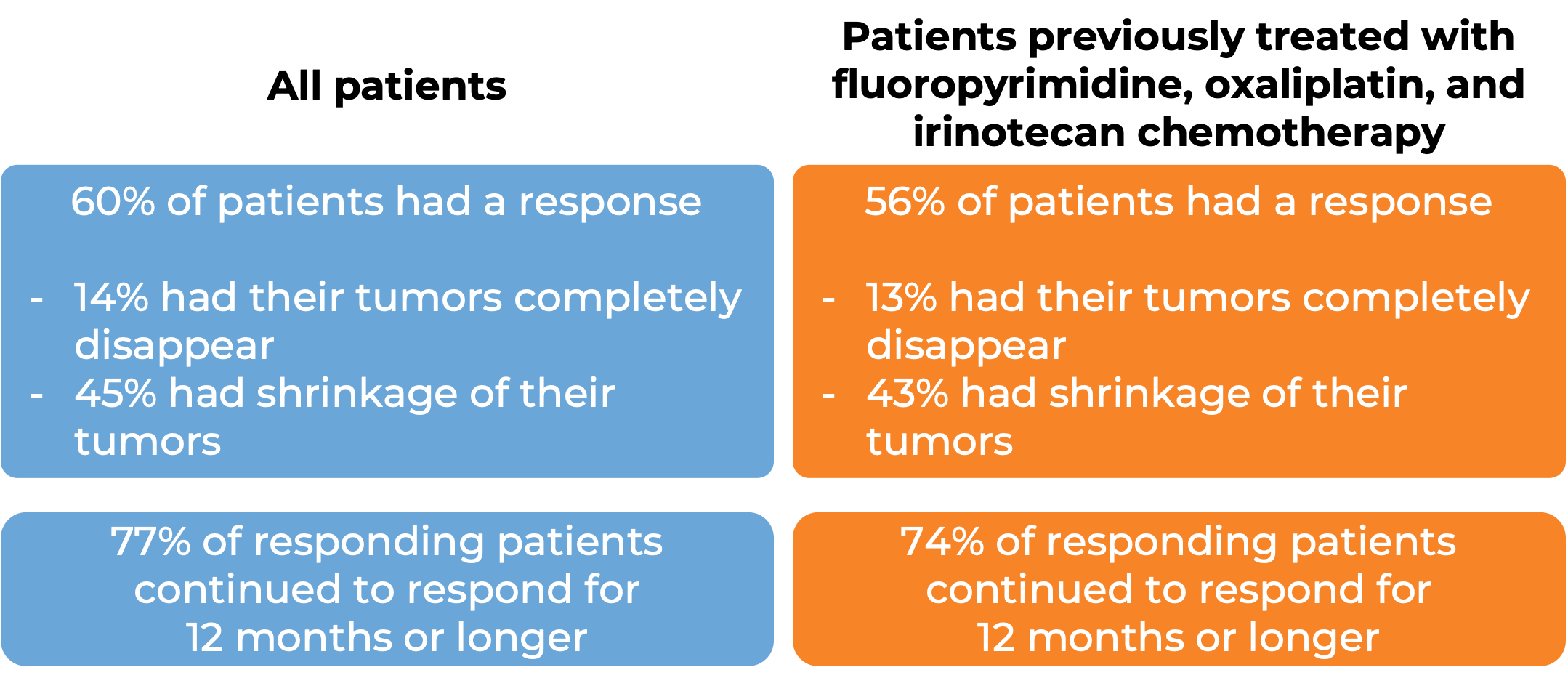
Note: Rounding percentages after the decimal can cause inconsistencies between the total and the sum of the parts.
Advanced liver cancer
In a clinical study, 49 patients with advanced liver cancer who had been treated with sorafenib (Nexavar), and it either did not work, stopped working, or was not tolerated, were treated with Opdivo plus ipilimumab (Yervoy). At a minimum follow-up of 28 months:
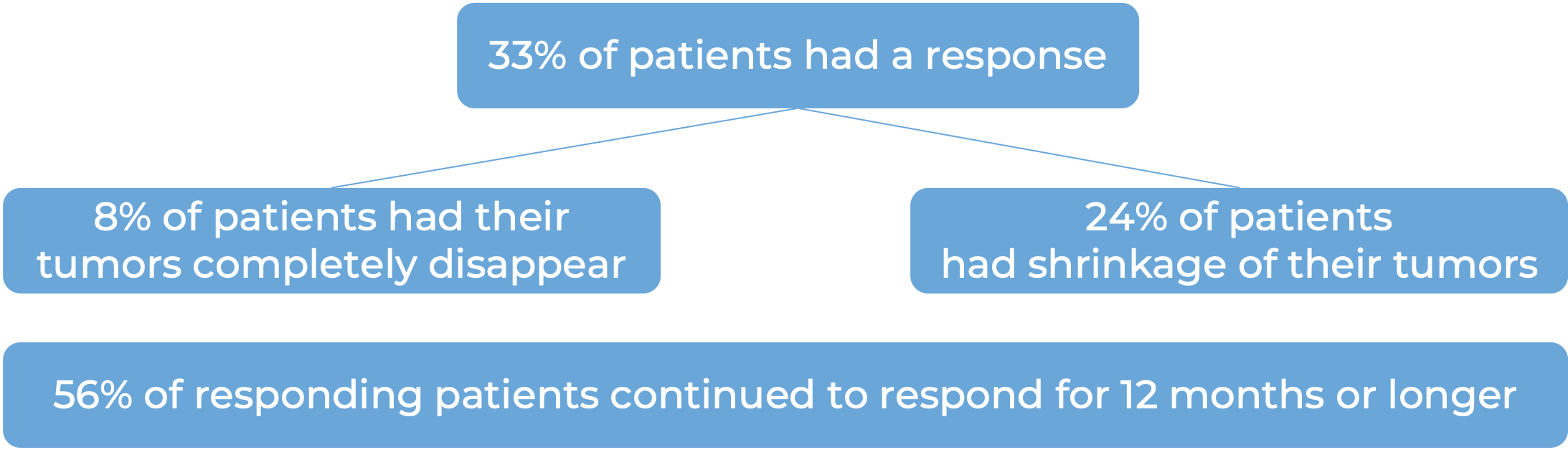
Note: Rounding percentages after the decimal can cause inconsistencies between the total and the sum of the parts.
Advanced esophageal squamous cell carcinoma
In another clinical study, 794 patients with esophageal or gastroesophageal junction cancer that had been treated with chemotherapy and concurrent radiation and was then removed by surgery, but who still had cancer cells remaining in their body after treatment, were treated with either Opdivo or a placebo.
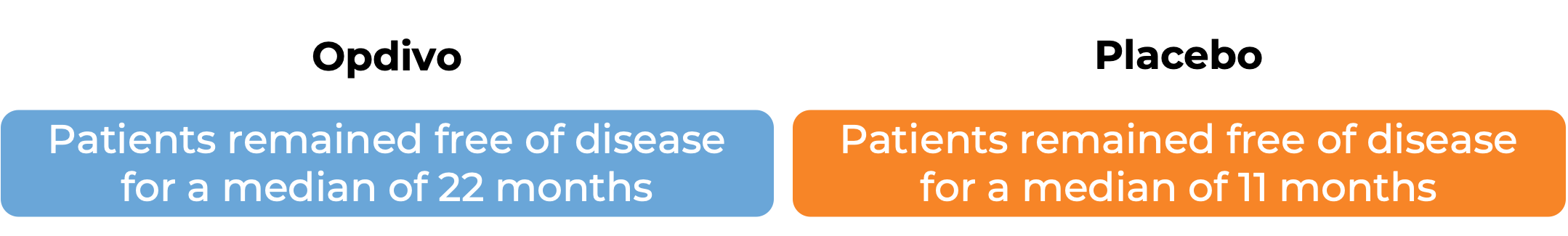
In a clinical study, 970 patients with previously untreated esophageal squamous cell carcinoma that could not be removed by surgery, recurred after surgery, or had spread to other parts of the body, were treated with either:
- Opdivo plus chemotherapy (fluorouracil and cisplatin),
- Opdivo plus ipilimumab (Yervoy), OR
- chemotherapy alone.
At a minimum follow-up of 13 months, among 473 patients whose tumor cells tested positive for the PD-L1 molecule:
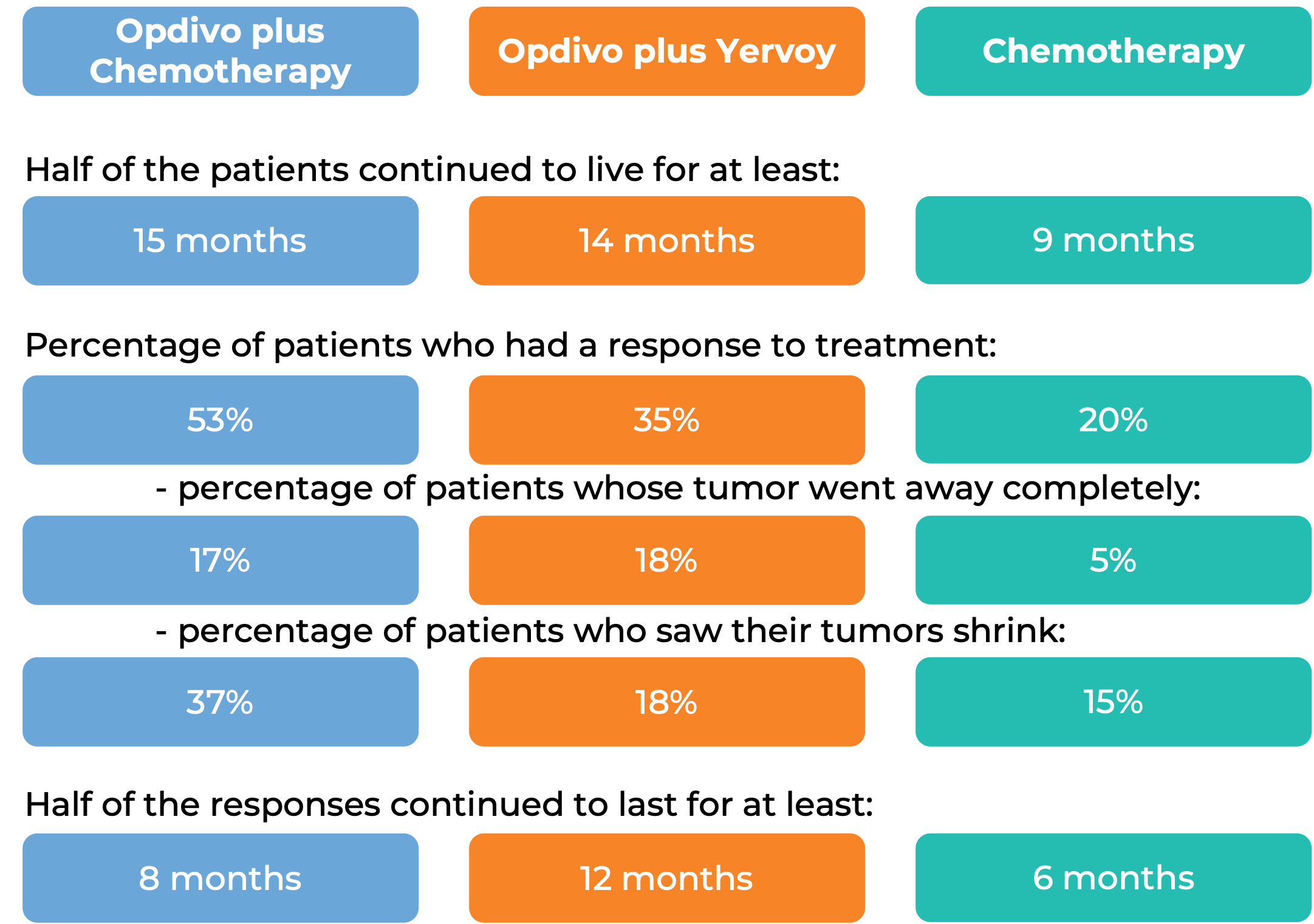
Note: Rounding percentages after the decimal can cause inconsistencies between the total and the sum of the parts.
In another clinical study, 419 patients with esophageal squamous cell carcinoma whose cancer had spread or come back and could not be removed by surgery, and who had been treated with chemotherapy containing fluoropyrimidine and platinum, but did not respond to or could not tolerate this treatment, were treated with either Opdivo or investigator’s choice of chemotherapy (docetaxel or paclitaxel). At a minimum follow-up of 18 months:
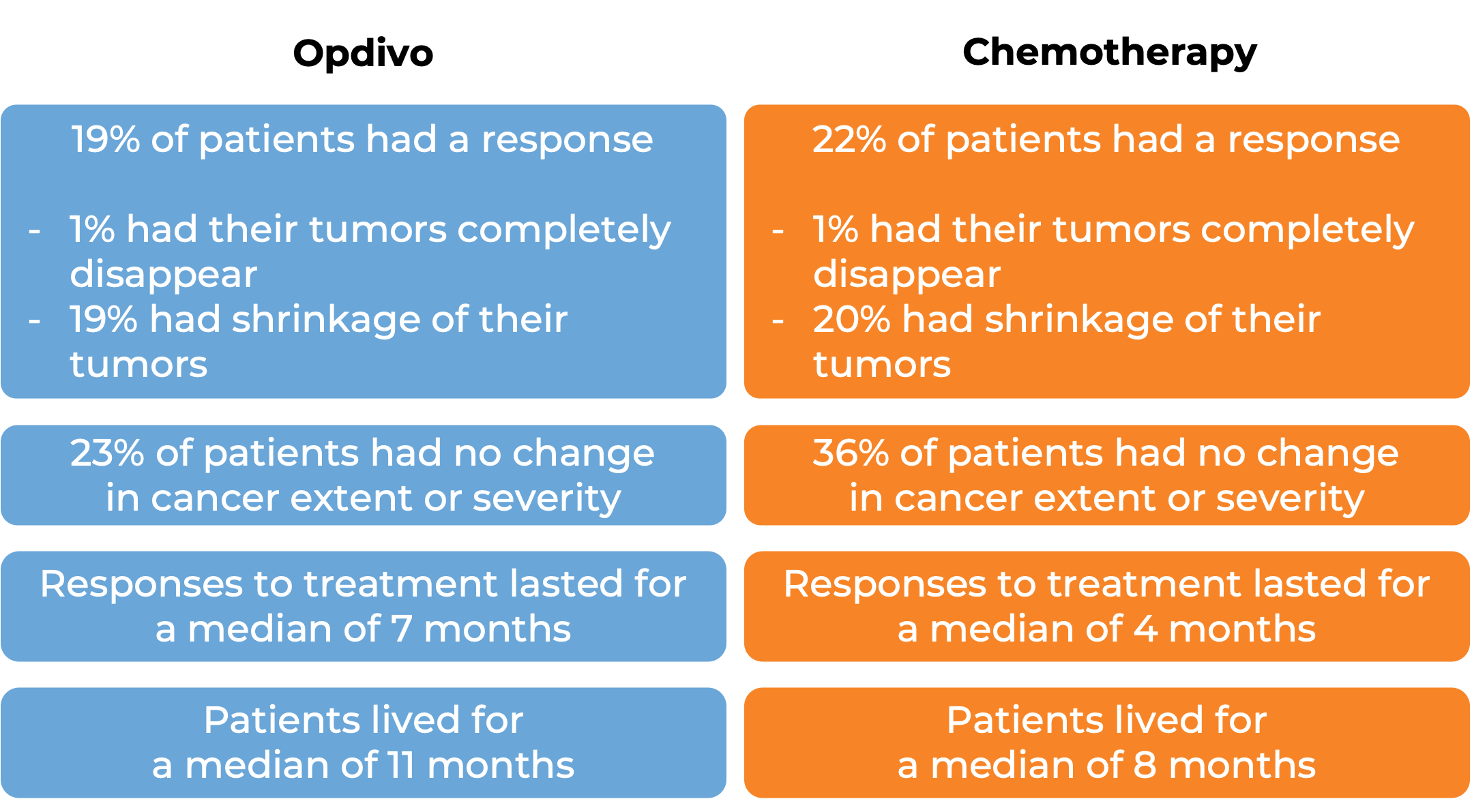
(For the definition of “median”, click HERE.) Note: Rounding percentages after the decimal can cause inconsistencies between the total and the sum of the parts.
Advanced stomach cancer
In a clinical trial, 1581 patients with previously untreated advanced stomach (gastric), gastroesophageal junction, or esophageal adenocarcinoma, were treated with either Opdivo in combination with chemotherapy (mFOLFOX6 [fluorouracil, leucovorin, and oxaliplatin] or CapeOX [capecitabine and oxaliplatin]) or chemotherapy alone. At a minimum follow-up of 12 months, among 1296 patients whose tumors tested positive for the PD-L1 molecule:
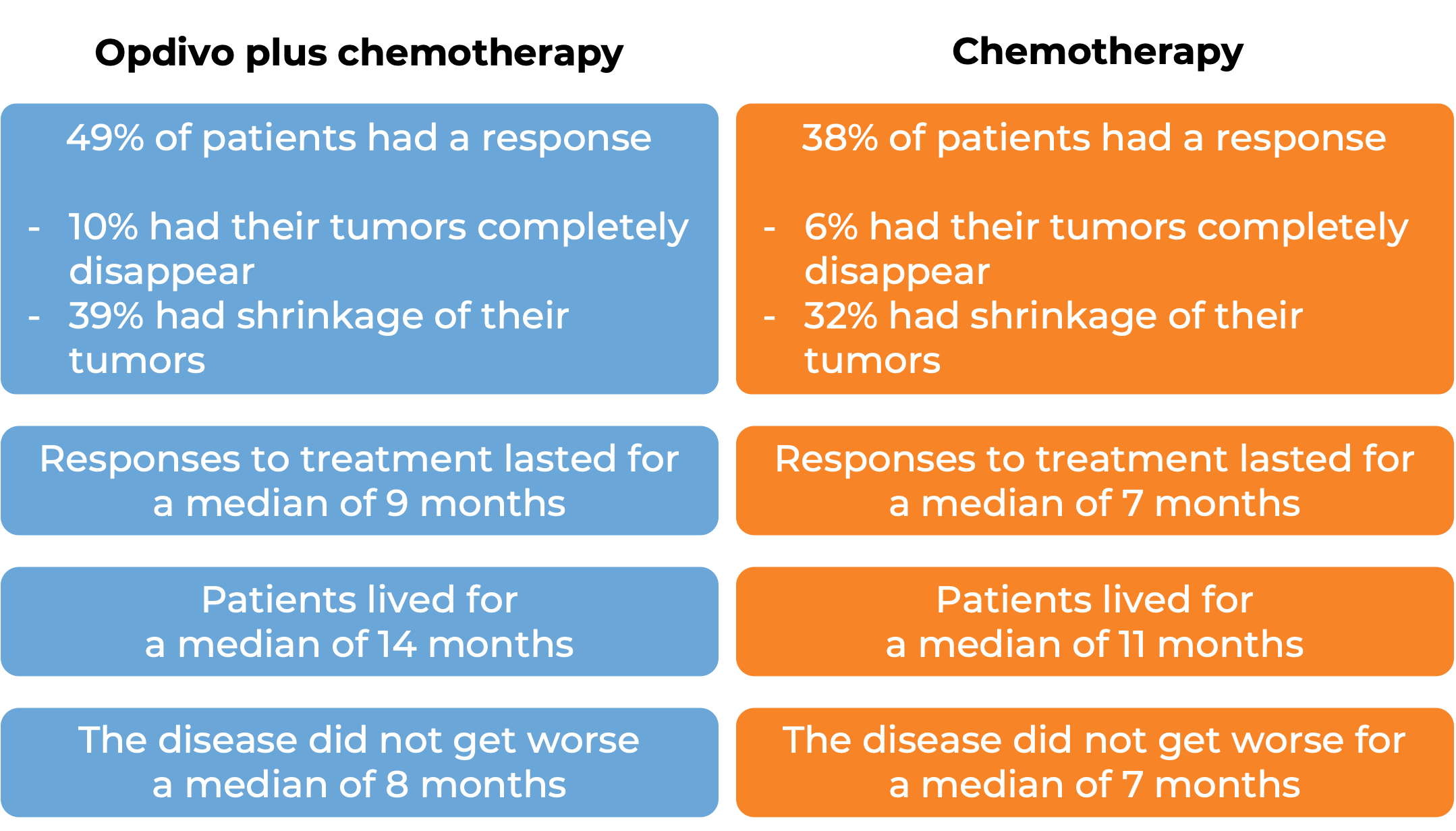
(For the definition of “median” click HERE.)
What are the side effects?
The most common side effects of Opdivo Qvantig include pain (in the muscles, bones, joints, back, and stomach areas), fatigue, itching, rash, underactive thyroid, diarrhea, cough, and abdominal pain. Based on the side effects of Opdivo, common side effects may also include headache, constipation, nausea, vomiting, decreased appetite, shortness of breath, upper respiratory tract or urinary tract infections, and fever.
Opdivo Qvantig can cause the patient’s T cells to attack healthy cells throughout the body. Because of this, Opdivo Qvantig can cause side effects that can become serious or life-threatening, and may lead to death. Some of the serious side effects related to Opdivo Qvantig include inflammation of the lungs, liver, brain, kidneys (which can lead to kidney failure), or colon (which can result in tears or holes in the intestine). Additionally, problems can arise with hormone glands (including thyroid, pituitary, and adrenal glands, as well as the pancreas). Opdivo Qvantig may cause Type 1 diabetes. Skin rash (which could become severe and life-threatening), and reactions related to the infusion may also occur. Patients should report any symptoms to their healthcare provider who can then initiate actions to limit or reverse the side effects.
Patients may experience other side effects when Opdivo Qvantig is used in combination with other treatments. For a more complete list of possible side effects, see the full prescribing information.
Additional information
Manufacturer
Bristol‑Myers Squibb
Approval
FDA and EMA (but different indications, this document is for FDA only)
Links to drug websites
- US: https://www.opdivo.com/subcutaneous-injection
- Europe: https://www.ema.europa.eu/en/medicines/human/EPAR/opdivo
Other references
- Nivolumab monotherapy in patients with advanced platinum-resistant urothelial carcinoma: Efficacy and safety update from CheckMate 275.Siefker-Radtke AO, Baron AD, et al. Journal of Clinical Oncology (2019)
- https://news.bms.com/press-release/corporatefinancial-news/us-food-and-drug-administration-approves-opdivo-nivolumab-ye-0
- https://ascopost.com/news/september-2020/checkmate-9er-trial-shows-benefit-of-nivolumabcabozantinib-for-advanced-kidney-cancer/
- Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. Bajorin DF, et al. The New England Journal of Medicine (2021)
- Nivolumab in Previously Untreated Melanoma without BRAF Mutation. Robert C, et al. The New England Journal of Medicine (2015)
- Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. Doki Y, et al. The New England Journal of Medicine (2022)
- Adjuvant nivolumab in resected stage IIB/C melanoma: primary results from the randomized phase 3 CheckMate 76K trial. [Kirkwood JM, et al. Nature Medicine (2023)](<* https://www.nature.com/articles/s41591-023-02583-2>)
- Adjuvant Nivolumab versus Ipilimumab in Resected Stage III/IV Melanoma: 5-Year Efficacy and Biomarker Results from CheckMate 238. Larkin J, et al. Clinical Cancer Research
- Nivolumab plus Gemcitabine-Cisplatin in Advanced Urothelial Carcinoma. Van der Heijden MS, et al. The New England Journal of Medicine (2023)
- Nivolumab plus cabozantinib versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended follow-up from the phase III randomised CheckMate 9ER trial. Powles T, et al. ESMO Open (2024)
- Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. Choueiri TK, et al. The New England Journal of Medicine (2021)
- Perioperative Nivolumab in Resectable Lung Cancer. Cascone T, et al. The New England Journal of Medicine (2024)
Last updated: July 11, 2025


