How is this drug name pronounced?
Nogapendekin alfa inbakicept: noh-GAP-en-DEH-kin AL-fuh in-BAK-ih-sept
Anktiva: ank-TEE-vuh
What cancer(s) does this drug treat?
Bladder cancer
Anktiva is approved for:
- Patients with non-muscle invasive bladder cancer (NMIBC) in which the cancer cells are confined to the lining of the bladder (carcinoma in situ) that does not respond to Bacillus Calmette-Guerin (BCG) treatment. In such cases, Anktiva is used in combination with BCG.
Limitations of use
Age: The safety and efficacy of Anktiva in patients under 18 years of age have not been established.
Pregnancy/Breastfeeding: Anktiva may cause harm to a fetus, and is not recommended for use during pregnancy. Patients who can become pregnant should use contraception during treatment with Anktiva and for at least 1 week after the last dose of Anktiva. The risks associated with Anktiva during breastfeeding are not known, but levels of Anktiva outside of the bladder are low, indicating that transfer of the drug to breast milk would be limited. The benefits of breastfeeding and the patient’s need for treatment should be weighed when considering treatment with Anktiva.
What type of immunotherapy is this?
How does this drug work?
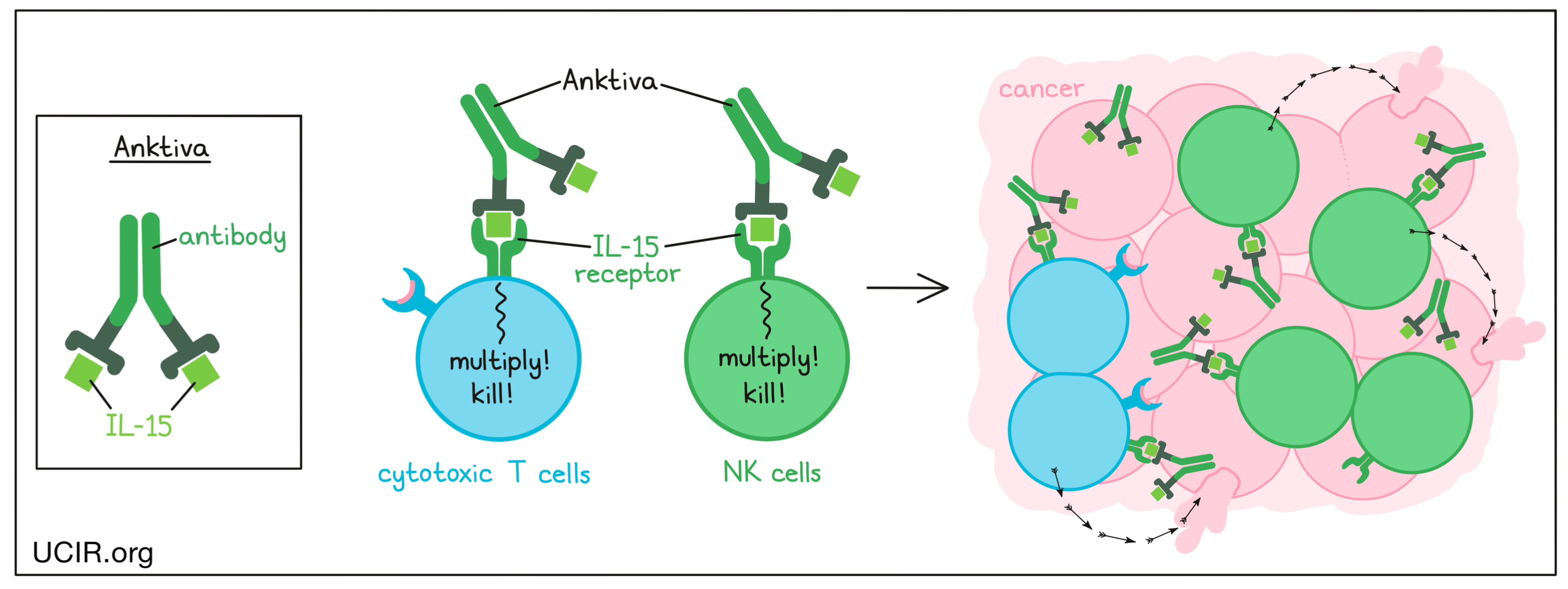
Target: IL-15 receptor (IL-15R)
Anktiva is a drug comprising a portion of an antibody fused to the cytokine IL-15. The IL-15 portion of Anktiva targets and engages with the IL-15 receptor, which is present on the surface of immune cells, including T cells and NK cells. When the IL-15 receptor is engaged by Anktiva, it signals the immune cells to multiply and become more activated, enhancing their ability to attack and kill cancer cells.
How is this drug given to the patient?
Aktiva is administered directly into the bladder through a urinary catheter. The catheter is then removed and the drug mixture is left in the bladder for 2 hours, unless the patient needs to void sooner. During this time, the patient may be repositioned at regular intervals to maximize the exposure of the entire bladder surface to Anktiva. Anktiva is administered once a week for six weeks, in combination with BCG. If the cancer is not gone by month 3, a second course of treatment may be given. Long-term Anktiva is given in combination with BCG once a week for 3 weeks at months 4, 7, 10, 13, and 19 to prevent the cancer from coming back. For patients who are still responding at 25 months, long-term treatment is given in combination with BCG once a week for 3 weeks at months 25, 31, and 37.
What are the observed clinical results?
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies.
Bladder cancer
In a clinical trial, 77 patients with non-muscle invasive bladder cancer with carcinoma in situ, that did not respond to BCG treatment, and for whom any amount of tumor that could be removed by surgery was removed, but the patient had a high risk for the cancer to return or get worse, were treated with Anktiva plus BCG. Overall, 62% of patients responded to treatment, with 58% of responses lasting a year or longer, and 40% of responses lasting two years or longer.
What are the side effects?
The most common side effects of Anktiva include painful urination, blood in the urine, increased frequency and urgency of urination, urinary tract infections, pain in the bones or muscles, chills, and fever. Abnormal blood test results, including increased creatinine and potassium, were also common.
Delaying surgery to remove all or part of the bladder increases the risk of the bladder cancer spreading into the thick muscle in the bladder wall and to other parts of the body. Patients with carcinoma in situ who do not respond to treatment with Anktiva plus BCG after their second induction course (second treatment once a week for 6 weeks) should reconsider surgical removal of all or part of the bladder.
Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects. For a more complete list of possible side effects, see the full prescribing information.
Additional information
Manufacturer
AGC Biologics
Approval
FDA
Links to drug websites
- US: https://anktiva.com/hcp
- Europe: N/A
Last updated on May 29, 2025


