How is the drug name pronounced?
Polatuzumab Vedotin: paul-oh-TOO-zoo-MAB ved-AUGHT-in
Polivy: PAUL-i-VIE
What cancer(s) does this drug treat?
Diffuse large B-cell lymphoma (DLBCL)
Polivy is approved for:
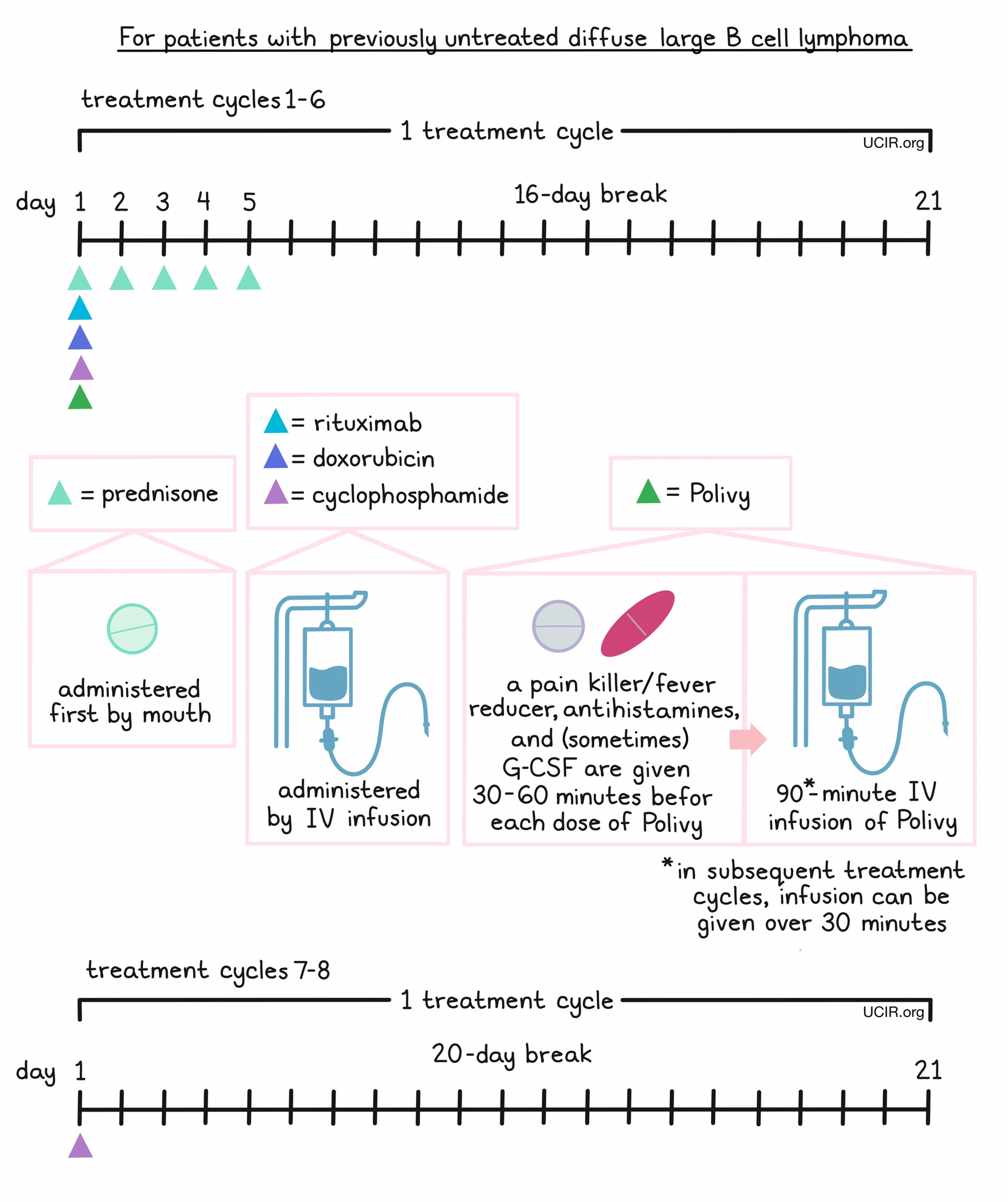
- Patients with previously untreated diffuse large B-cell lymphoma, not otherwise specified (NOS), or high-grade B-cell lymphoma (HGBL), who have an International Prognostic Index score of 2 or greater. In such cases, Polivy is given in combination with a rituximab product (e.g., Rituxan or Truxima) and cyclophosphamide, doxorubicin, and prednisone (R-CHP) chemotherapy.
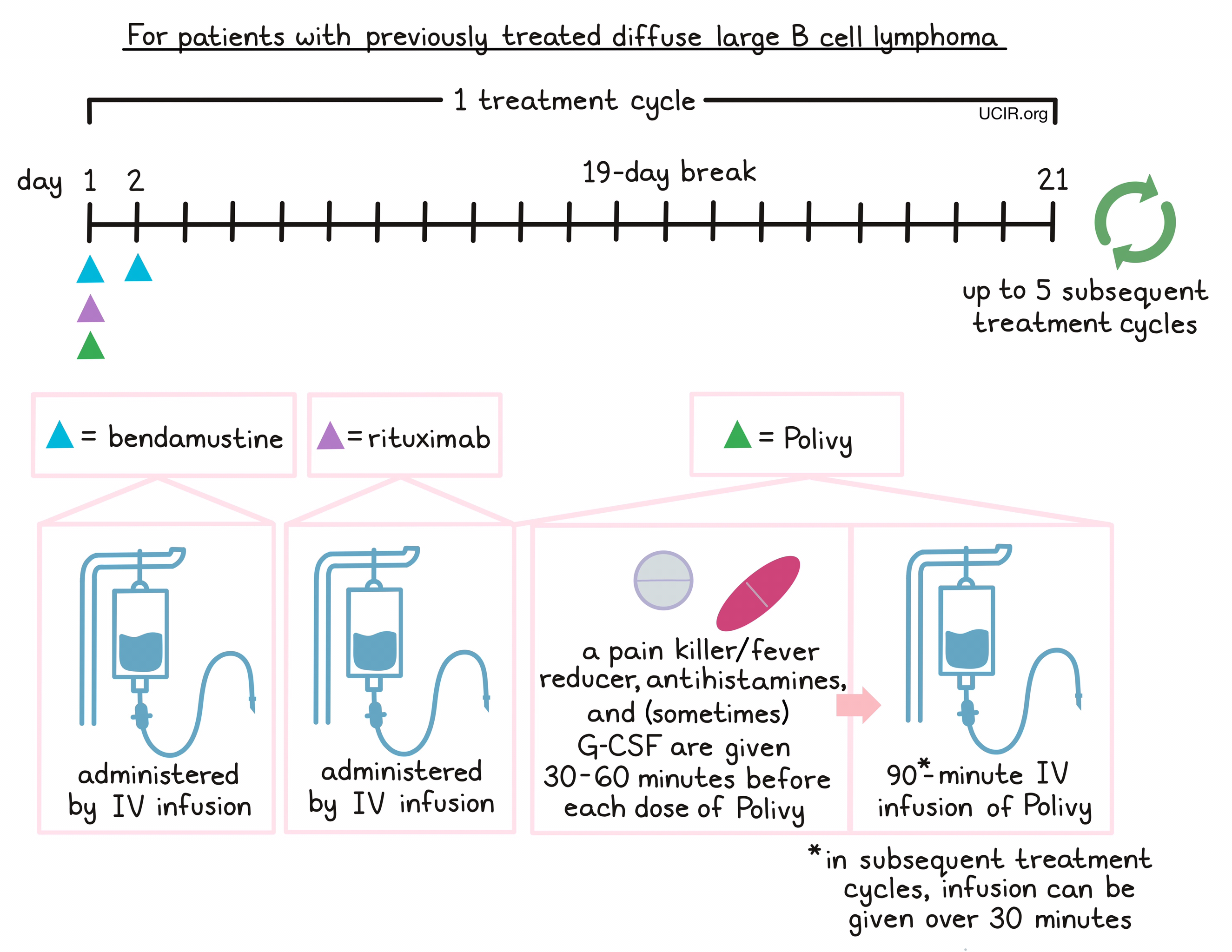
- Patients with diffuse large B-cell lymphoma, not otherwise specified (NOS), who have been previously treated with at least two different therapies, but whose disease either did not respond to the treatment (refractory disease) or has since returned (relapsed disease). In such cases, Polivy is given in combination with the chemotherapy bendamustine and a rituximab product (e.g., Rituxan or Truxima).
Limitations of Use
Age: The safety and efficacy of Polivy has not been established in patients under 18 years of age.
Fertility/Pregnancy/breastfeeding: Polivy may impair fertility in men. In pregnant women, Polivy can cause harm to the embryo and fetus, and is not recommended for use during pregnancy. Women should not start Polivy treatment when pregnant. Women are advised to use contraception during treatment with Polivy and for at least 3 months after the last dose of Polivy. Men are advised to use contraception during treatment with Polivy and for at least 5 months after the last dose of Polivy. The risks associated with Polivy during breastfeeding are not known and cannot be ruled out. Due to the potential for serious adverse reactions in the breastfed child, women are advised not to breastfeed during treatment with Polivy and for up to 2 months after the last dose of Polivy.
Interactions with other drugs: Polivy should not be administered at the same time as medications called CYP3A inhibitors or CYP3A inducers.
What type of immunotherapy is this?
- Antibody–drug conjugate
How does this drug work?
Target:
- CD79b molecule on B cells
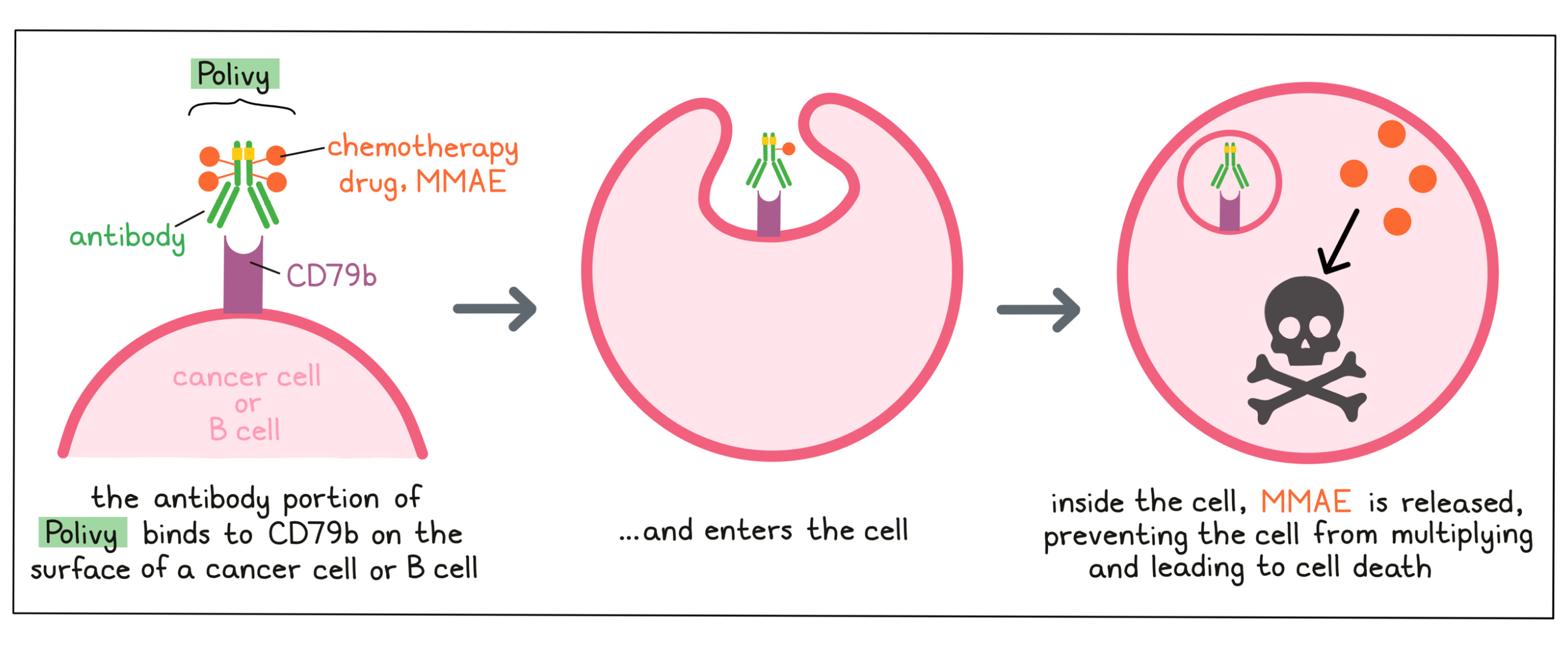
Polivy is an antibody–drug conjugate that was made in the laboratory by joining a small, highly potent chemotherapy drug to a large antibody molecule. The antibody delivers the chemotherapy drug to a cancer cell, reducing the effects of the chemotherapy drug on most other cells of the body.
Antibody molecules are proteins that can bind tightly to a particular target molecule. Antibodies have an overall “Y” shape. The two tips of the upper arms of the “Y” shape are the parts of the antibody that can very precisely bind to their targets. The target molecule that the antibody in Polivy binds to is CD79b, a molecule which is commonly found on the surface of cancer cells in patients with diffuse large B-cell lymphoma.
The chemotherapy drug Monomethyl auristatin E (MMAE) is joined to the stem of the antibody’s “Y” shape. There are about 4 MMAE molecules bound to every antibody molecule in Polivy. MMAE is not toxic when it is joined to the antibody, and needs to be released in order to become active.
When Polivy binds to CD79b on the surface of cancer cells, it can enter the cell it is bound to. Inside the cell, the chemotherapy part of Polivy is released and becomes activated. MMAE prevents the cancer cells from multiplying and leads to their death.
Because CD79b is also present on most healthy B cells, Polivy may attack these cells and cause unwanted effects, in addition to killing cancer cells.
How is this drug given to the patient?
Polivy is administered via a tube in the vein (intravenous infusion, or I.V.) over 90 minutes on day 1. If the first infusion of Polivy is well tolerated, subsequent infusions can be given over 30 minutes. Polivy is given in 21-day intervals. Each interval is one “treatment cycle”, and patients may receive up to 6 treatment cycles.
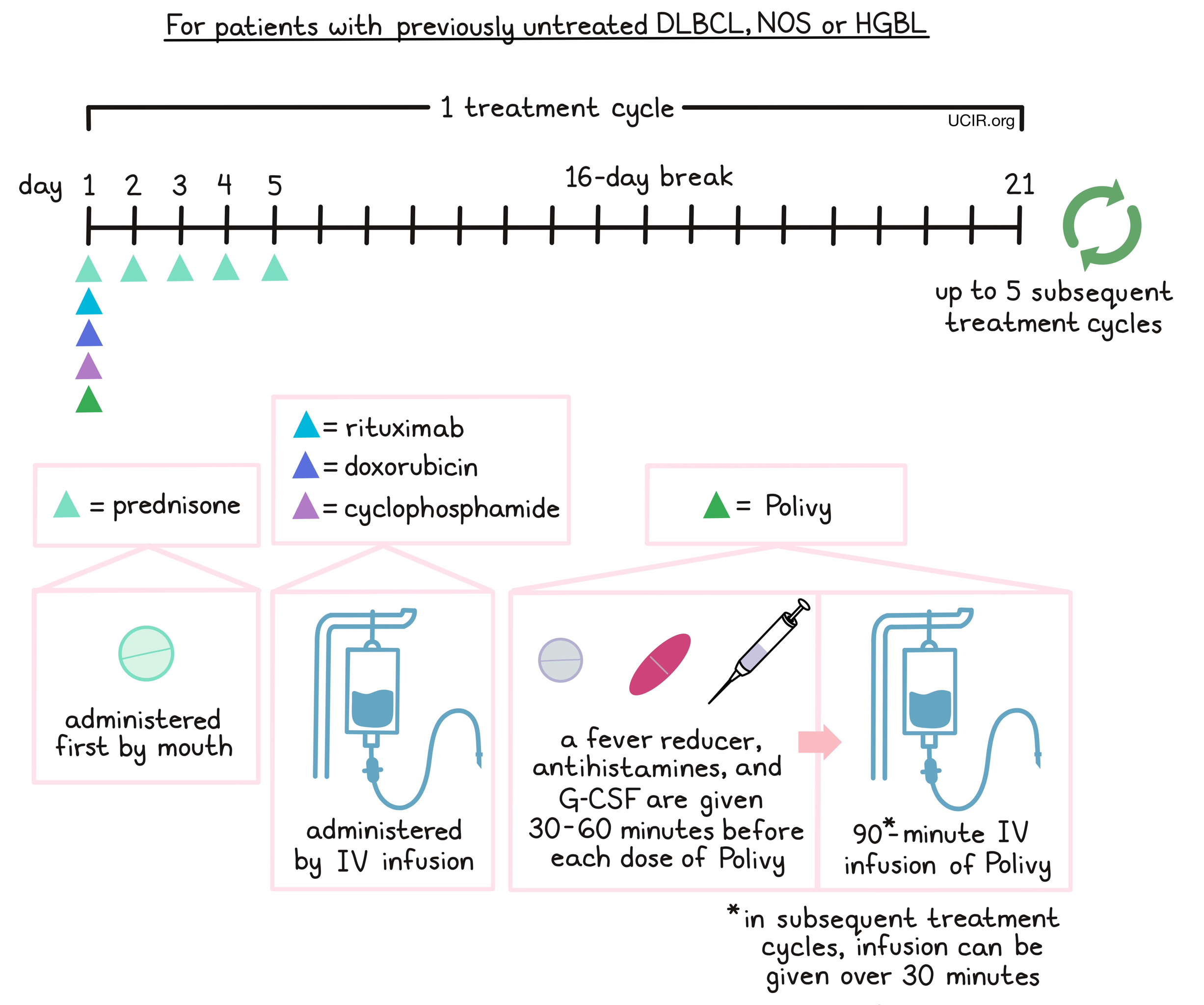
For patients with previously untreated DLBCL, NOS or HGBL, prednisone is given on day 1, followed by Polivy, cyclophosphamide, doxorubicin, and a rituximab product in any order. 30 to 60 minutes prior to each administration of Polivy, patients receive a pain reliever and fever reducer (such as acetaminophen) and an antihistamine (such as diphenhydramine) to help reduce the chance of a reaction. Patients are also given an injection of G-CSF to prevent neutropenia (low neutrophil counts). Prednisone is then given again by itself on days 2-5, followed by a 16-day break.
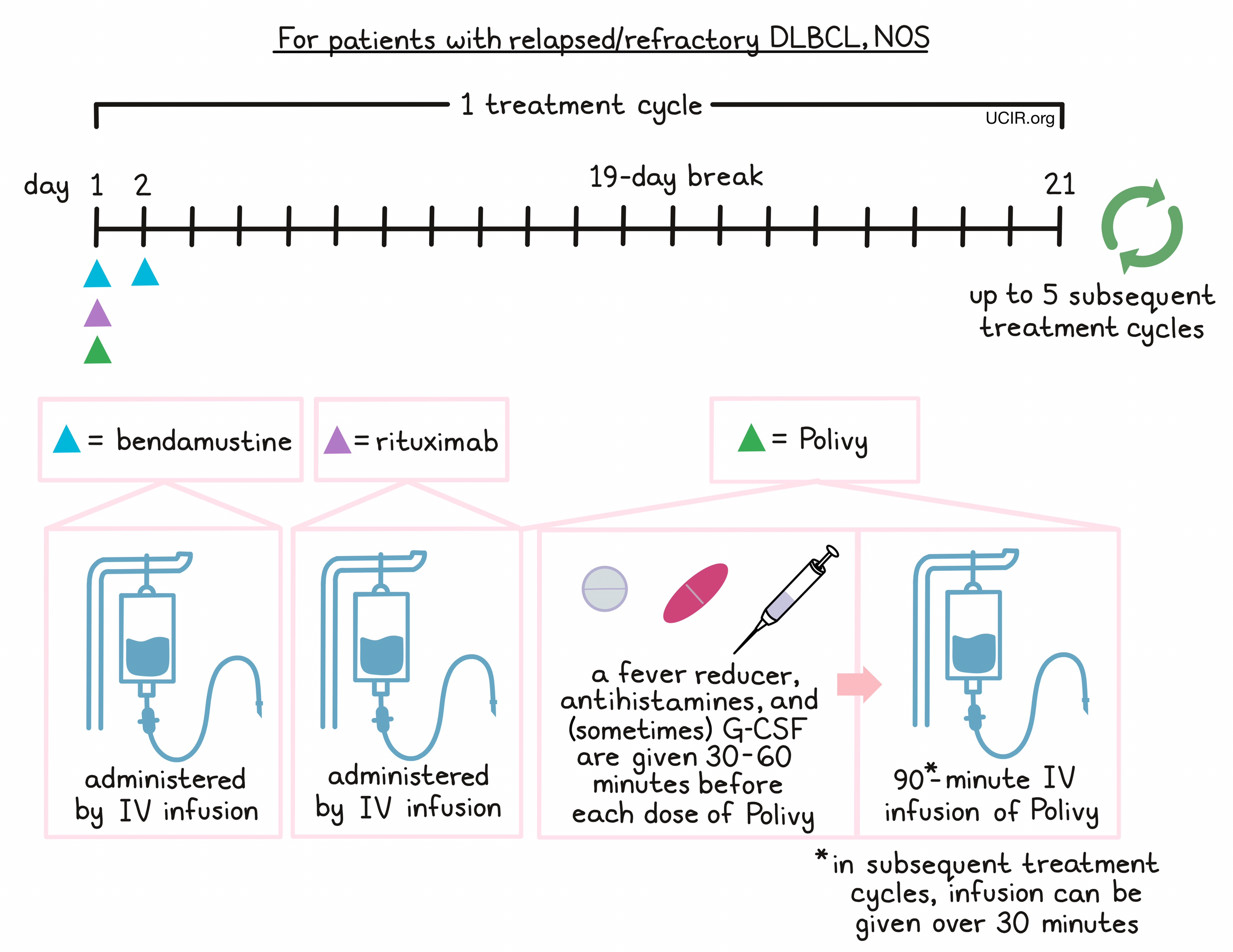
For patients with relapsed or refractory DLBCL, NOS, Polivy is given in combination with bendamustine and rituximab in any order on day 1. 30 to 60 minutes prior to each administration of Polivy, patients receive a pain reliever and fever reducer (such as acetaminophen) and an antihistamine (such as diphenhydramine) to help reduce the chance of a reaction to the infusion. Patients may also be given an injection of G-CSF to prevent neutropenia (low neutrophil counts). Bendamustine is then given again by itself on day 2, followed by a 19-day break.
Administration of Polivy does not require a hospital stay. Patients are observed for any symptoms related to the infusion for at least 90 minutes after the first dose, and for 30 minutes after subsequent doses of Polivy. If any severe side effects or reactions occur, the infusion is immediately interrupted and only started again after symptoms subside.
Patients with a high amount of cancer in their body may receive additional treatment prior to receiving Polivy to prevent tumor lysis syndrome, a serious side effect caused by the rapid breakdown of cancerous cells. Before and during Polivy treatment, patients also receive medications to prevent pneumonia that is caused by a fungal infection, and medications to prevent herpes virus infections.
What are the observed clinical results?
For:
Diffuse large B-cell lymphoma (previously untreated) Diffuse large B-cell lymphoma (previously treated)
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Diffuse large B-cell lymphoma (previously untreated)
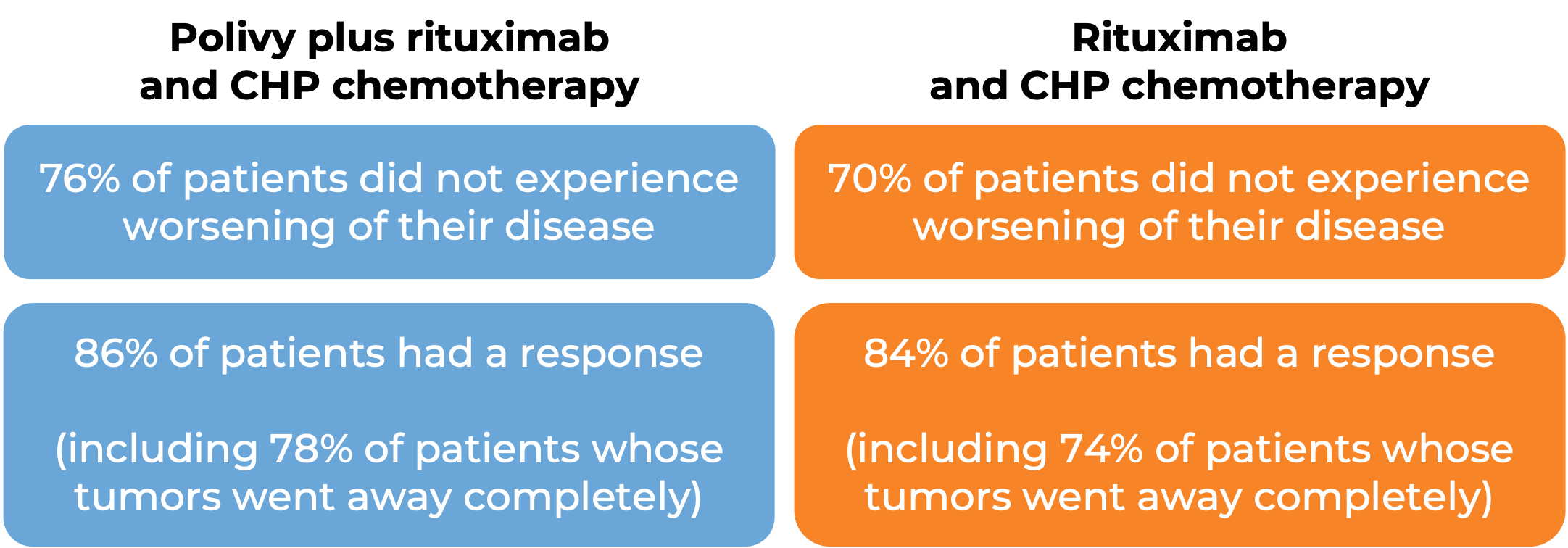
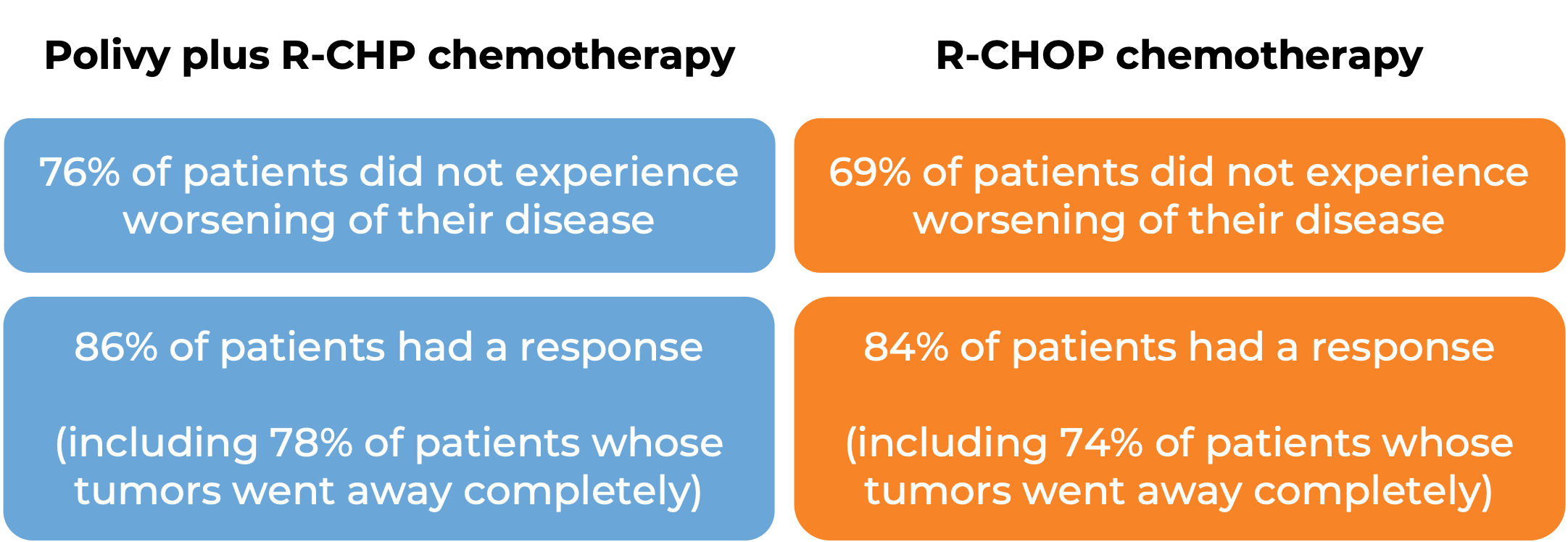
In a clinical trial, 435 patients with previously untreated diffuse large B-cell lymphoma, not otherwise specified (NOS), or high-grade B-cell lymphoma (HGBL), were treated with Polivy and rituximab (e.g., Rituxan or Truxima) and R-CHP (cyclophosphamide, doxorubicin, and prednisone chemotherapy) or R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone). At a median follow-up of 25 months:
Diffuse large B-cell lymphoma (previously treated)
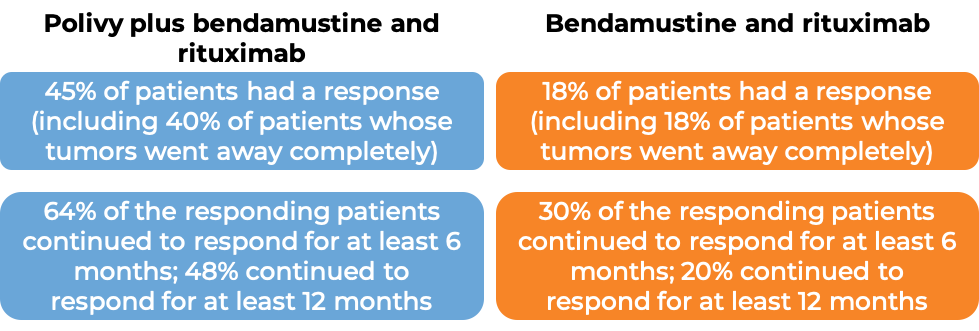
In a clinical trial, 80 patients with diffuse large B-cell lymphoma who had been previously treated, but whose disease either did not respond or had come back, were treated with Polivy, bendamustine, and a rituximab product (e.g., Rituxan or Truxima), or with bendamustine and a rituximab product alone. At end of treatment (6-8 weeks after day 1 of the last treatment cycle):
(For the definition of “median”, click HERE.)
What are the potential side effects?
Polivy targets the CD79b molecule, which, while present on cancerous B cells, is also present on normal B cells. As a result, Polivy can kill normal B cells, increasing the risk of serious or life-threatening bacterial, fungal, or viral infections, including pneumonia, upper respiratory tract infections, and sepsis.
The most common side effects of Polivy include nerve problems in the arms and legs (such as changes in touch, numbness, tingling, pain, and muscle weakness), reactions to the Polivy infusion, changes in the blood (such as low white blood cell counts, low red blood cell counts, and low platelet counts), tiredness, dizziness, nausea, vomiting, diarrhea, fever, decreased appetite, weight loss, and blurred vision. In rare cases, Polivy can seriously damage the liver.
Patients should immediately report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects. For a more complete list of possible side effects, see the full prescribing information.
Additional Information
Manufacturer
Genentech (a member of the Roche group)
Approval
EMA and FDA
Links to drug websites
Last updated on August 11, 2025