How is this drug name pronounced?
Sacituzumab Govitecan: SAK-ih-TOO-zoo-mab GOH-vih-TEE-kan
Trodelvy: troh-DEL-vee
What cancer(s) does this drug treat?
Advanced breast cancer
Trodelvy is approved for:
- Patients with triple-negative breast cancer (cancer that tests negative for estrogen hormone receptor, progesterone hormone receptor, and human epidermal growth factor receptor 2 [HER2]) that has spread to other parts of the body (metastatic), and cannot be removed by surgery, and who have received at least two prior therapies, one of which was for their advanced disease.
- Patients with breast cancer that has grown and cannot be removed by surgery or has spread to other parts of the body, tests positive for estrogen hormone receptor, and tests negative for human epidermal growth factor receptor 2 (HER2). Patients must have been previously treated with therapy that stops the effect of estrogen on breast cancer and at least two other therapies for their advanced disease.
Limitations of use:
Age: The safety and efficacy of Trodelvy in patients under 18 years of age have not been established.
Fertility/Pregnancy/Breastfeeding: Trodelvy may impair fertility in women. Trodelvy may cause harm (including death) to the fetus and is not recommended for use during pregnancy. Women should not start Trodelvy treatment when pregnant and are advised to use contraception during treatment with Trodelvy and for at least 6 months after the last dose of Trodelvy. Men are advised to use contraception during treatment with Trodelvy and for at least 3 months after the last dose of Trodelvy. The risks associated with Trodelvy during breastfeeding are not known and cannot be ruled out. Due to the potential for serious adverse reactions in the breastfed child, women are advised not to breastfeed during treatment with Trodelvy and for at least 1 month after the last dose of Trodelvy.
Interactions with other drugs: Trodelvy should not be administered at the same time as medications called UGT1A1 inhibitors or UGT1A1 inducers. Patients with genetically predisposed reduced UGT1A1 activity are at an increased risk of adverse reactions to treatment with Trodelvy.
What type of immunotherapy is this?
- Antibody-drug conjugate
How does this drug work?
- Target: trophoblast cell-surface antigen-2 (Trop-2)
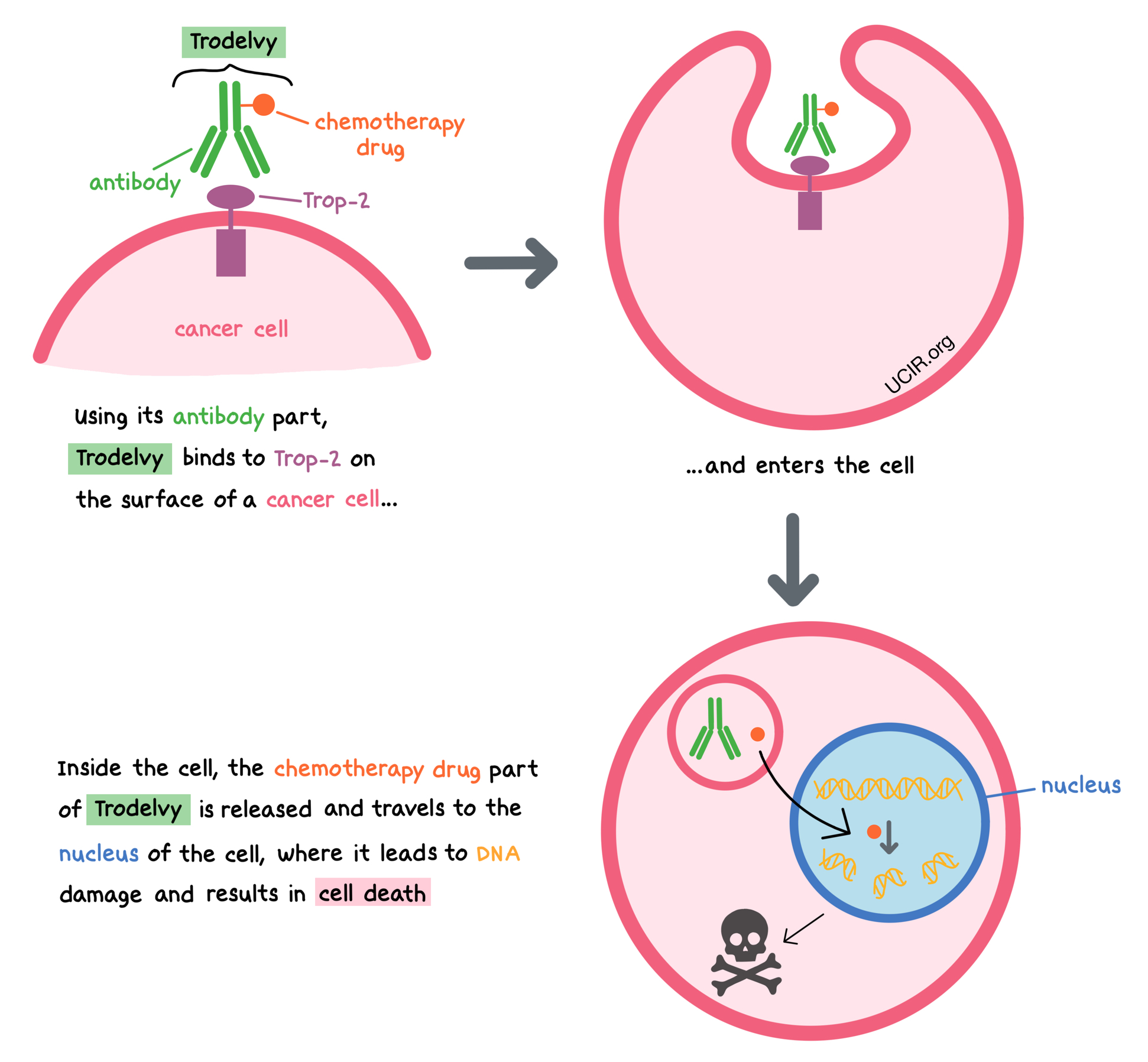
Trodelvy is a medicine that consists of two parts that are connected: an antibody that attaches to a molecule called trophoblast cell-surface antigen-2 (Trop-2) on the surface of breast cancer cells, and a chemotherapy drug that can kill cancerous cells.
Trop-2 is present on the surface of many normal cells; however, it is present in much higher quantities on the surface of many types of cancer cells, including triple-negative breast cancer cells. Trodelvy is designed to minimize harm to normal, healthy cells and to bring the chemotherapy directly to the cancer cells. When Trodelvy binds to Trop-2 and enters the cancer cell, the chemotherapy drug part of Trodelvy is released and travels to the nucleus of the cell, where it leads to DNA damage and results in cell death.
How is this drug given to the patient?
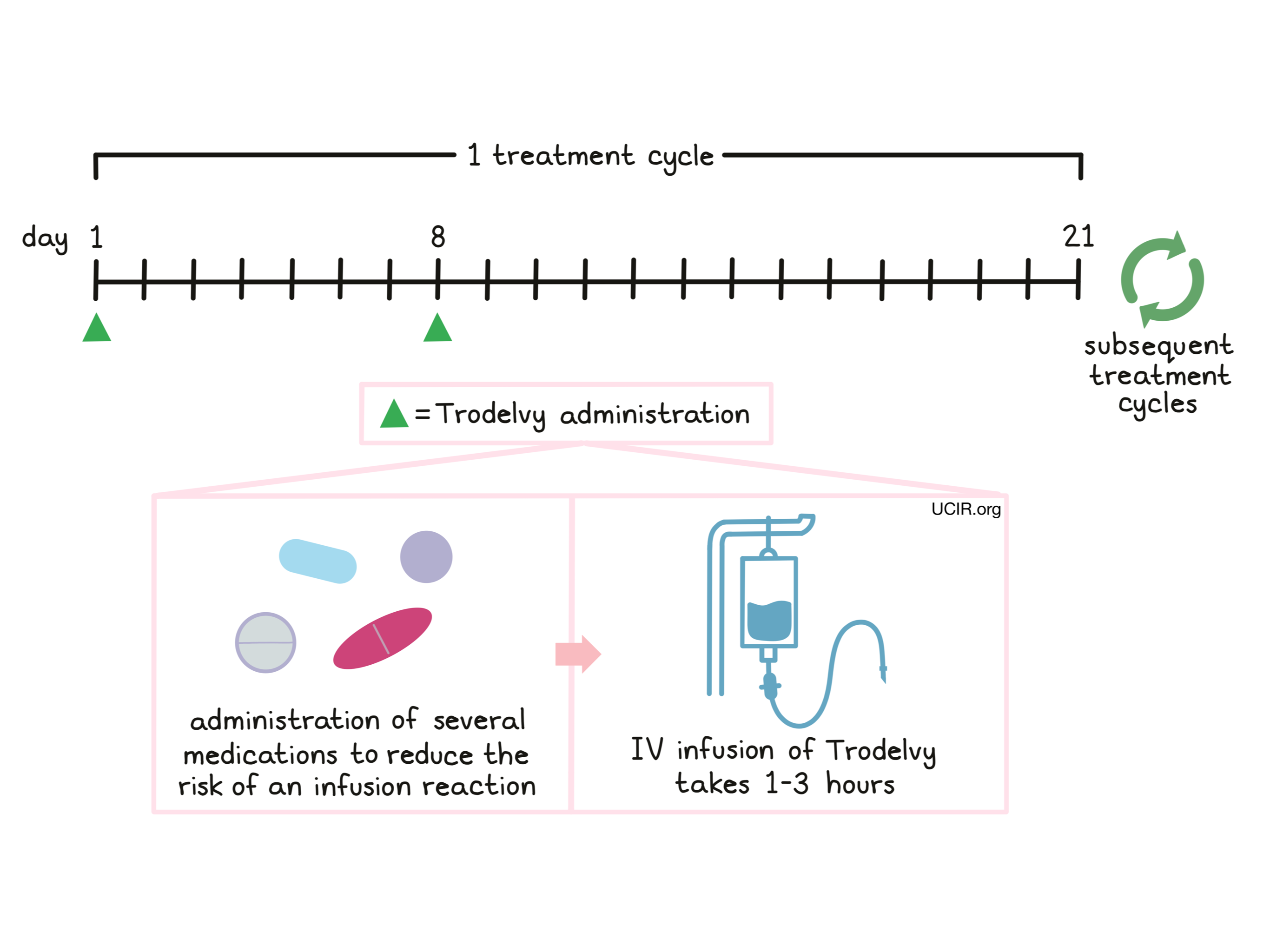
Patients receive several medications prior to each dose of Trodelvy to help reduce the chance of a reaction to the infusion and to help prevent chemotherapy-induced nausea and vomiting:
- Antihistamines
- Acetaminophen
- A corticosteroid
- An antiemetic (to prevent nausea and vomiting)
Trodelvy is administered via a tube into a vein (intravenous infusion, or i.v.) over three hours on days 1 and 8, followed by 13 days without treatment. Each 21-day (3-week) period is one “treatment cycle”. The total number of treatment cycles depends on how well the patient responds to the treatment. If the first dose is well tolerated, subsequent doses may be administered over 1 to 2 hours. The healthcare provider decides how long the treatment with Trodelvy will continue.
Before and periodically during the treatment, blood tests are performed to monitor for side effects. Patients may also receive medications to prevent infections, and G-CSF, a treatment that helps to prevent low white blood cell counts.
What are the observed clinical results?
For:
Advanced triple-negative breast cancer
Advanced hormone receptor-positive, HER2-negative breast cancer
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies.
Advanced triple-negative breast cancer
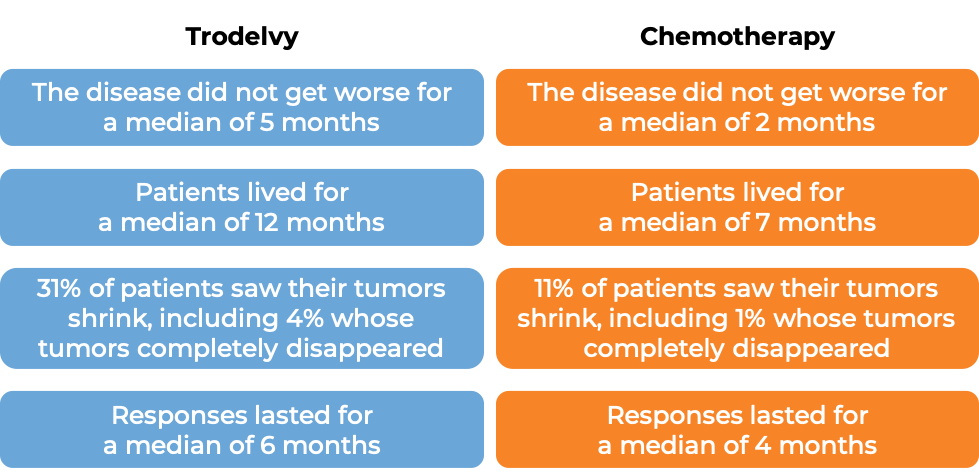
In a clinical trial, 108 patients with triple-negative breast cancer that had spread to other parts of the body (metastatic), and who had received at least two prior therapies for their metastatic disease were treated with Trodelvy. At a median follow-up of 10 months:
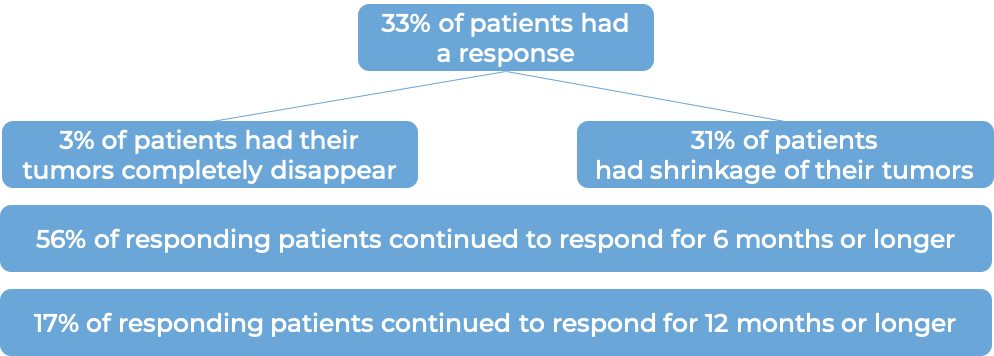
In another clinical trial, 529 patients with triple-negative breast cancer that had grown within the breast and could not be removed by surgery, or had spread to other parts of the body, and who had received at least two prior chemotherapies for their advanced disease were treated with Trodelvy or a chemotherapy treatment of their physician’s choice (eribulin, capecitabine, gemcitabine, or vinorelbine).
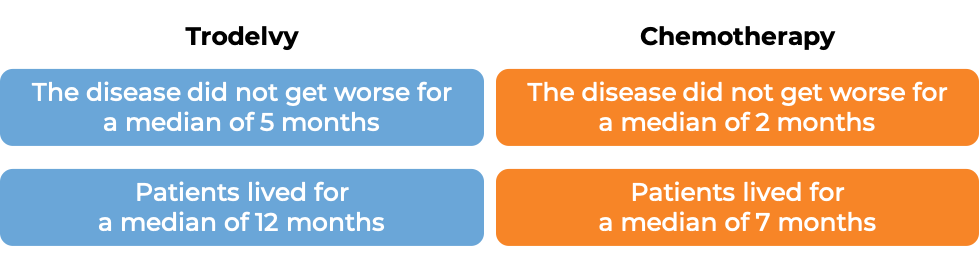
(For the definition of “median”, click HERE.)
Advanced hormone receptor-positive, HER2-negative breast cancer
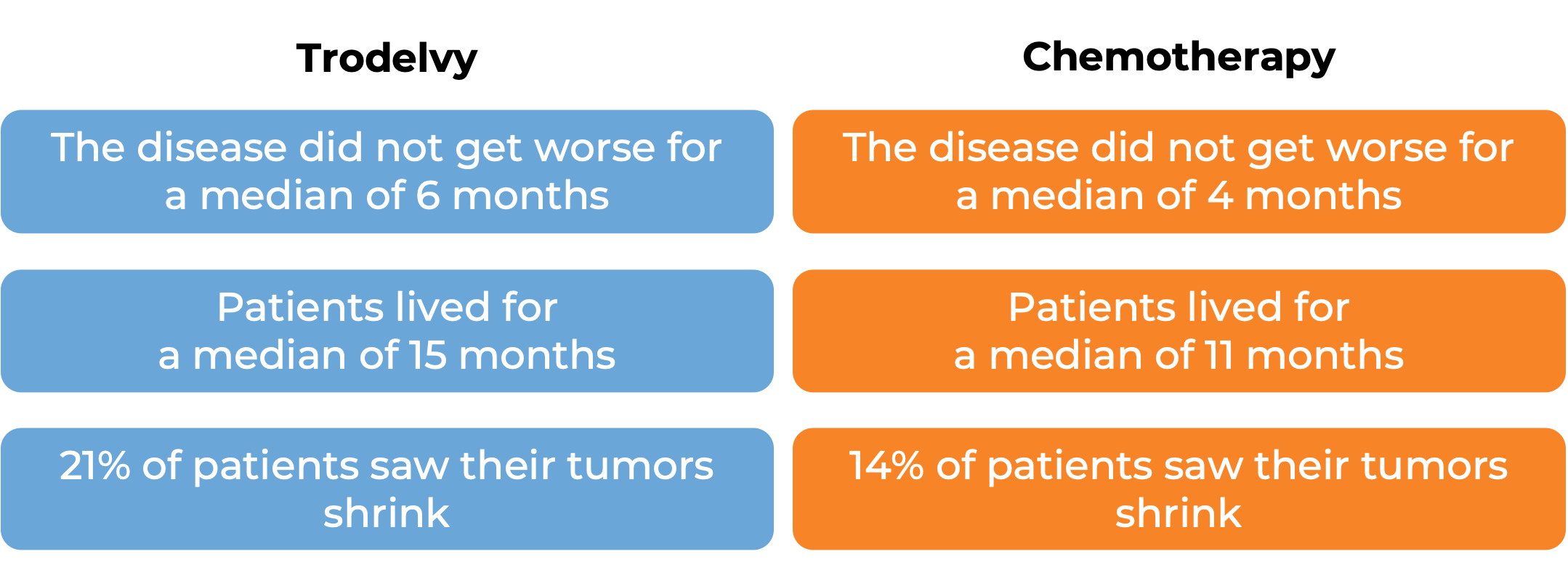
In a clinical trial, 543 patients with previously treated breast cancer that had grown and could not be removed by surgery or had spread to other parts of the body, and tested positive for estrogen hormone receptor and negative for human epidermal growth factor receptor 2 (HER2), were treated with Trodelvy or a chemotherapy treatment of their physician’s choice (eribulin, capecitabine, gemcitabine, or vinorelbine). At a median follow-up of 13 months:
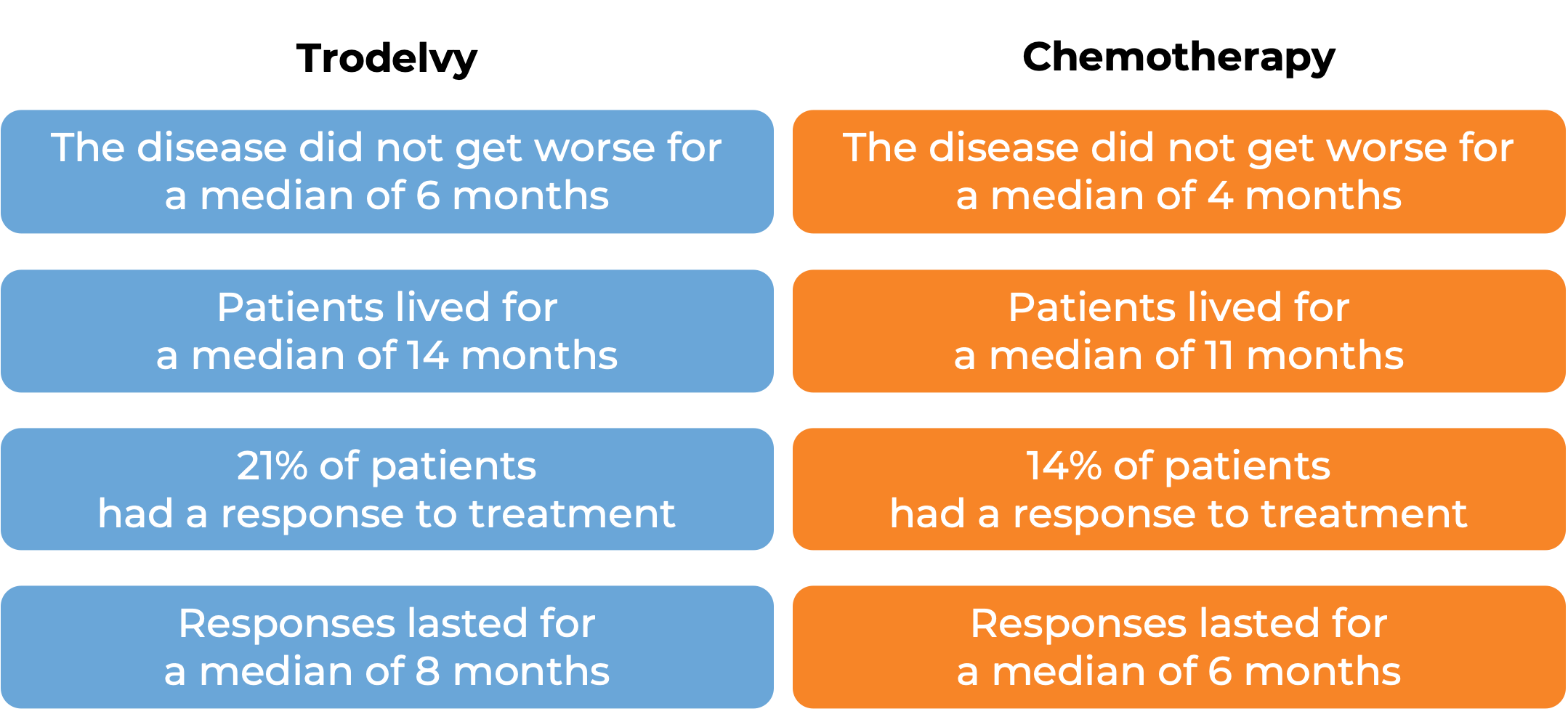
What are the side effects?
The most common side effects of Trodelvy include tiredness, hair loss, constipation, diarrhea, nausea and vomiting, decreased appetite, pain in the abdomen (stomach area), rash, decreased white and red blood cell counts, and other abnormal blood test results.
Trodelvy can cause side effects that can become serious or life-threatening and may lead to death. Some of the serious side effects related to Trodelvy include severe and life-threatening allergic reactions or reactions related to the infusion, severe nausea and vomiting, severe diarrhea, and a low white blood cell (neutrophil) count that can lead to life-threatening infections.
Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects. For a more complete list of possible side effects, see the full prescribing information.
Additional information
Manufacturer
Gilead Sciences, Inc.
Approval
FDA and EMA
Links to drug websites
- US: https://www.trodelvy.com/patient
- Europe: https://www.ema.europa.eu/en/medicines/human/EPAR/trodelvy
Other references:
- Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. Bardia A, et al. The New England Journal of Medicine (2019)
- Overall survival (OS) results from the phase III TROPiCS-02 study of sacituzumab govitecan (SG) vs treatment of physician’s choice (TPC) in patients (pts) with HRD/HER2- metastatic breast cancer (mBC). Rugo HS, et al. Annal of Oncology (2022)
Last updated: August 7, 2025