How is this drug name pronounced?
Sipuleucel-T: SY-puh-LOO-sel-TEE
Provenge: PROH-venj
What cancer(s) does this drug treat?
Advanced prostate cancer
Provenge is approved for:
- Patients with prostate cancer that has spread to other parts of the body and does not respond to therapy that lowers the amount of male sex hormones in the body (“hormon therapy”), and who have minimal or no symptoms.
Limitations of use:
Age: The safety and efficacy of Provenge in patients under 18 years of age have not been established.
Other: Provenge should be used with caution in patients who are at higher risk for the formation of blood clots. The use of Provenge with chemotherapy or immunosuppressive medication has not been studied and is not recommended.
What type of immunotherapy is this?
How does this drug work?
- Target: prostatic acid phosphatase (PAP)
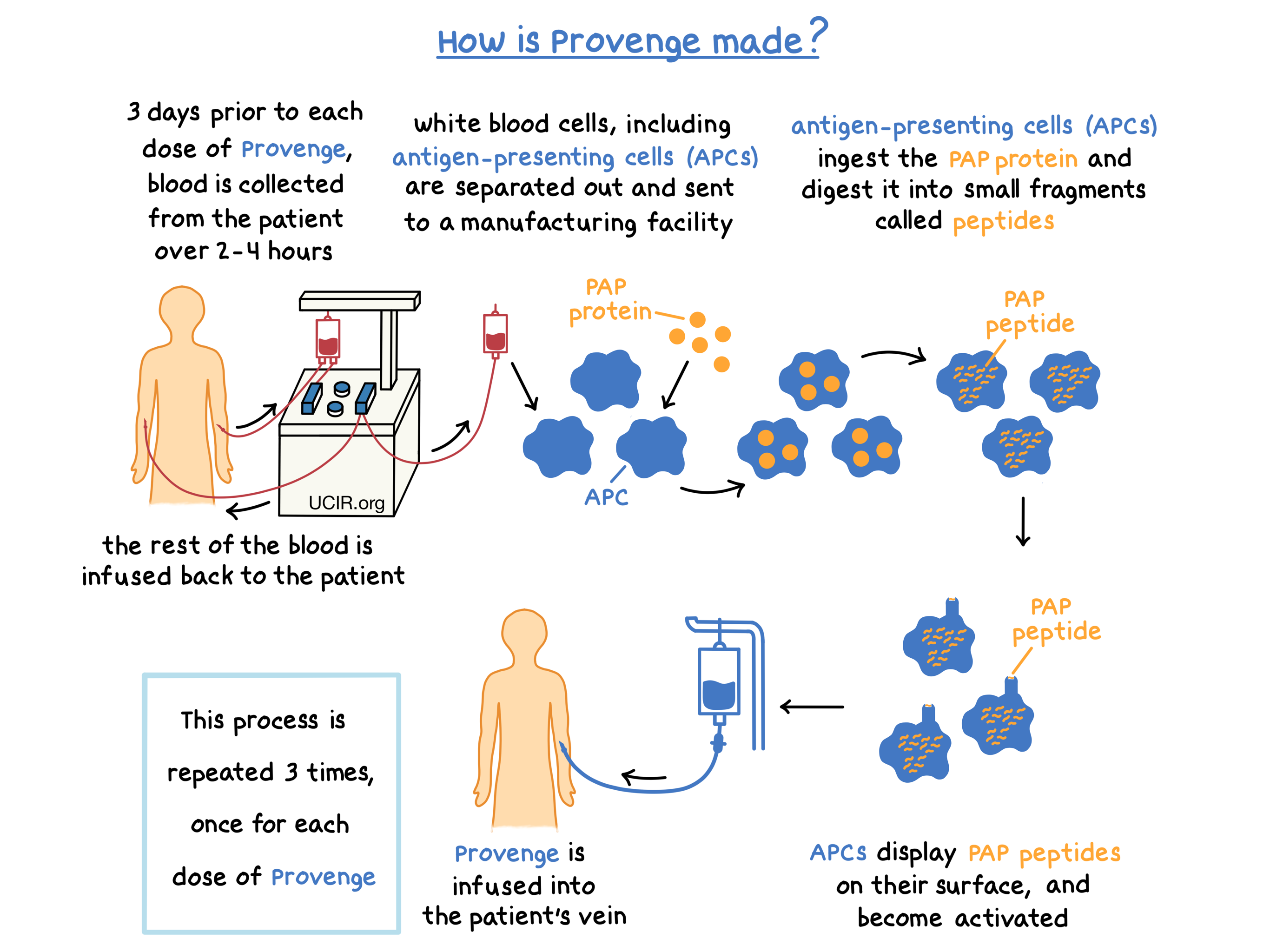
Provenge is made from a patient’s own white blood cells. Approximately 3 days prior to each dose of Provenge, patients undergo a process called leukapheresis, in which blood is collected from the patient’s vein, white blood cells are separated from the collected blood, and the rest of the blood is returned to the patient. Leukapheresis is performed at a cell collection center and takes about 2 to 4 hours. The collected white blood cells are then sent to a manufacturing center in order to make a personalized dose of Provenge.
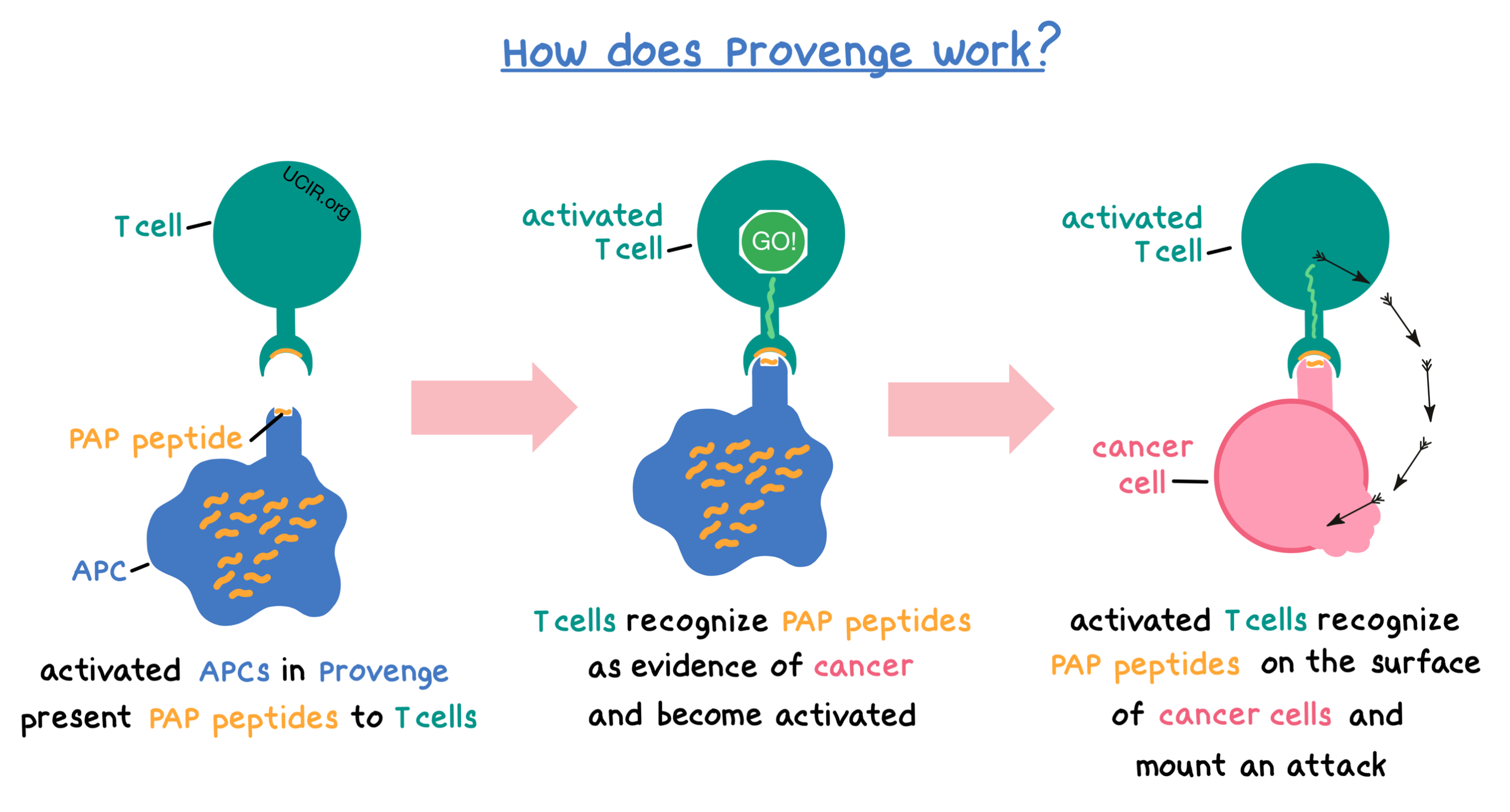
The collected white blood cells contain several types of immune cells, including antigen-presenting cells (APCs). During the process of manufacturing Provenge, a laboratory-made version of the protein called prostatic acid phosphatase (PAP), which is found in prostate cancer tissue at higher levels than in normal prostate tissue, and an immune cell activator (called GM-CSF) are added to the collected white blood cells. The APCs take up and digest the PAP protein into smaller fragments called peptides, which are then displayed on the surface of the APCs. At the same time, the APCs become activated.
When Provenge is administered to the patient, the peptides displayed on the surface of activated APCs can be recognized by other immune cells, such as T cells, which can then become activated and mount an attack against the cancer.
How is this drug given to the patient?
Approximately 30 minutes prior to receiving Provenge, patients receive acetaminophen and an antihistamine, such as diphenhydramine, to reduce the chance of reactions to the administration of Provenge.
Provenge is administered via a tube into a vein (intravenous infusion, or I.V.) over 1 hour once every two weeks for a total of three doses. Patients are monitored for at least 30 minutes after the infusion for any reactions.
What are the observed clinical results?
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Advanced prostate cancer
In a clinical trial, 512 patients with prostate cancer that had spread to other parts of the body and did not respond to hormone therapy, and who were not taking narcotics for pain related to their cancer, were treated with either Provenge or a control (white blood cells that were collected from the patient but were not activated in the laboratory). Patients receiving Provenge lived for a median of 26 months, compared with 22 months for patients in the control group.
In another clinical trial, 127 patients with prostate cancer that had spread to other parts of the body and did not respond to hormone therapy, and who had no cancer-related pain, were treated with either Provenge or a control (white blood cells that were collected from the patient, but were not activated in the laboratory). Patients receiving Provenge lived for a median of 26 months, compared with 21 months for patients in the control group.
What are the side effects?
The most common side effects of Provenge include chills, fever, fatigue, nausea, back pain, joint ache, and headache.
Provenge can cause side effects that can become serious or life-threatening. Some of the serious side effects related to Provenge include reactions related to the Provenge infusion, low blood pressure, blood clots, blockage of a blood vessel, problems with the lungs, heart (including heart attack), and brain (including stroke).
Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects. For a more complete list of possible side effects, see the full prescribing information.
Additional information
Manufacturer
Dendreon Pharmaceuticals
Approval
FDA
Links to drug websites
Last updated on January 16, 2021


