How is this drug name pronounced?
Talquetamab-tgvs: Tal-KAY-tah-mab
Talvey: TAL-vee
What cancer(s) does this drug treat?
Multiple Myeloma
Talvey is approved for:
- Patients with multiple myeloma who have received at least four prior treatments for their disease, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody (e.g., [daratumumab [Darzalex]](https://ucir.org/immunotherapy-drugs/daratumumab) or [isatuximab-irfc [Sarclisa]](https://ucir.org/immunotherapy-drugs/isatuximab-irfc), but whose cancer either did not respond to treatment or has since come back.
Limitations of use
Limitations: Talvey should only be administered in a treatment center and by adequately trained healthcare professionals who can ensure proper monitoring and immediate management of side effects associated with the drug.
Age: The safety and efficacy of Talvey in patients under 18 years of age have not been established.
Pregnancy/Breastfeeding: Talvey is not recommended for use during pregnancy due to potential for harm to the fetus. Patients who can become pregnant are advised to use contraception during treatment with Talvey and for 3 months after the last dose of Talvey. The risks associated with Talvey during breastfeeding are not known and cannot be ruled out. Due to the potential for adverse reactions in the breastfed child, patients are advised not to breastfeed during treatment with Talvey and for 3 months after the last dose of Talvey.
Interactions with other drugs: Dose adjustments may become necessary for patients treated with certain CYP substrates at the start of treatment with Talvey.
What type of immunotherapy is this?
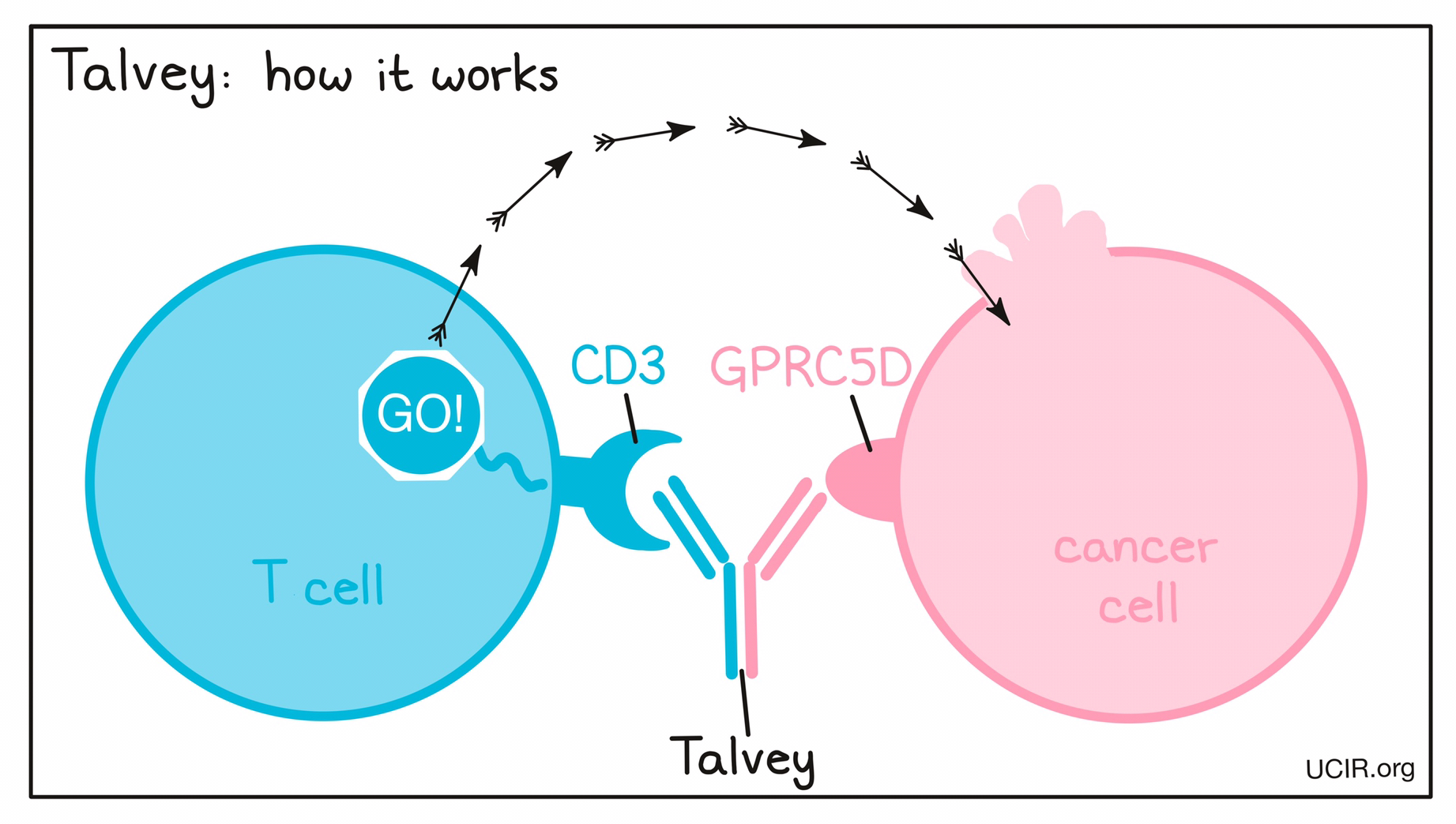
How does this drug work?
Targets:
- GPRC5D on multiple myeloma cells
- CD3 receptor on T cells
Talvey is a “bispecific antibody”. Antibodies are molecules that can bind to a particular target molecule. Bispecific antibodies are artificially made in the laboratory and can bind to two different targets at the same time. Talvey binds to:
- a molecule called GPRC5D on the surface of multiple myeloma cells. While GPRC5D is also present on some healthy cells, including plasma cells and epithelial cells in certain tissues, it is present at much higher quantities on the surface of cancer cells than normal healthy cells.
- a molecule called CD3 on the surface of T cells – the primary immune cells involved in killing cancer cells. CD3 is part of the T cell receptor, which is critical to the function of the T cell and is involved in stimulating the T cell to become active.
Because Talvey can bind to one molecule on multiple myeloma cells and another on T cells at the same time, it acts as a bridge and keeps the T cell in close contact with the cancer cell. By binding CD3 on the T cell, Talvey also stimulates the T cell to become activated and kill the cell it is bound to. Bispecific antibodies that direct T cells to kill cancer cells by binding to both cells at the same time are known as bispecific T cell engagers (BiTEs).
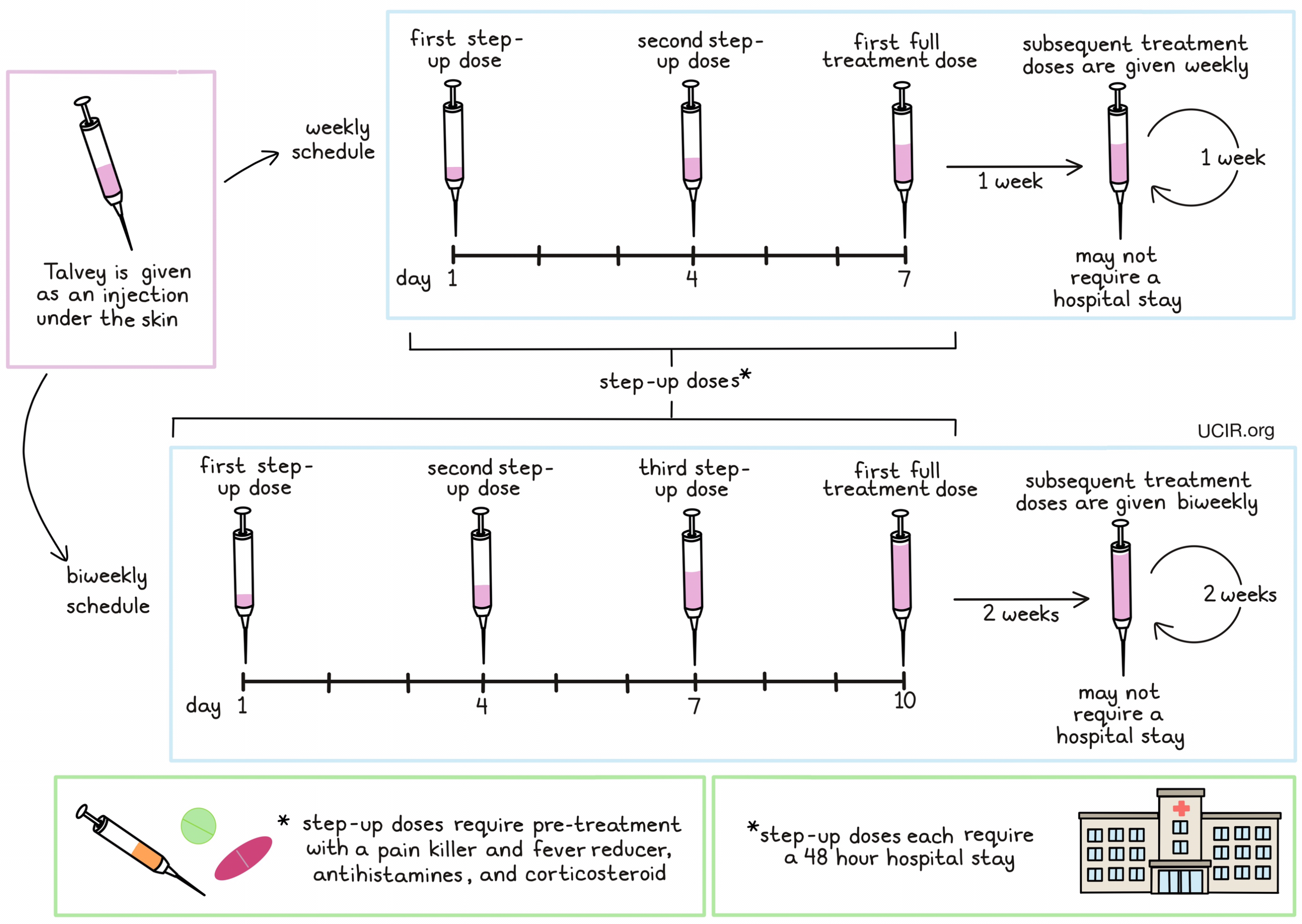
How is the drug given to the patient?
Talvey is given as an injection just below the skin (subcutaneous injection) in the lower part of the abdomen or the thigh. Patients receive Talvey on either a weekly or biweekly (every 2 week) basis. For patients on a weekly schedule, a small dose of Talvey is given on day 1, followed by larger dose on day 4, and a full-sized dose on day 7. For patients on a biweekly schedule, a small dose of Talvey is given on day 1, followed by larger dose on day 4, another larger dose on day 7, and a full-sized dose on day 10. This “step-up” dosing helps to reduce the risk of a reaction to Talvey. Additionally, during step-up dosing, patients receive several medications 1 to 3 hours prior to each dose of Talvey to help reduce the risk of cytokine release syndrome:
- A corticosteroid (dexamethasone)
- An antihistamine (diphenhydramine)
- A painkiller and fever reducer (acetaminophen)
Patients may continue to receive pre-treatment medications prior to full-sized doses of Talvey if dosing is delayed or if certain adverse reactions occur. During step-up dosing, each dose of Talvey requires a 48-hour hospital stay to monitor for potentially serious reactions to treatment.
What are the observed clinical results?
It is important to keep in mind that each patient’s outcome is individual and may be different from the results found in the clinical studies.
Multiple Myeloma
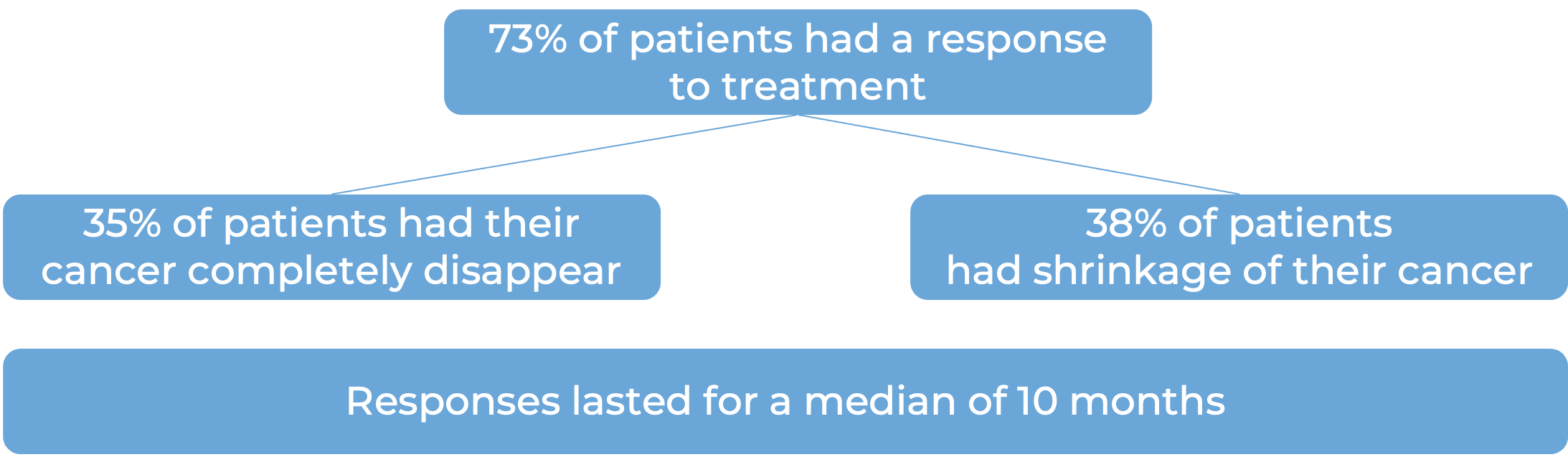
In a clinical study, 187 patients with multiple myeloma who had received at least three prior treatments for their disease, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody, but whose cancer either did not respond to treatment or had since come back, were treated with Talvey. 100 patients received weekly dosing with Talvey at a lower dose. The median time to first response was one month. At a median follow-up of 14 months from first response:
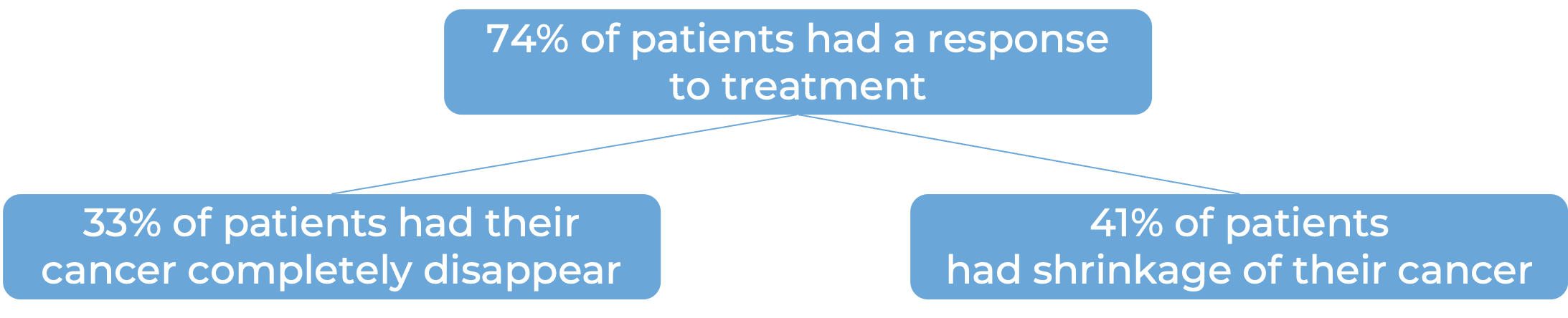
Further, 87 patients received biweekly (once every two weeks) dosing with Talvey at a higher dose. At a median follow-up of 6 months from first response among these patients:
What are the potential side effects?
Common side effects of Talvey include fever, cytokine release syndrome, an altered sense of taste, nail disorder, pain in the muscles, joints, and bones, skin problems (including rash, dry skin, redness, or bumps), fatigue, weight loss, dry mouth and/or eyes, difficulty swallowing, upper respiratory tract infection, diarrhea, low blood pressure, and headache. Some side effects, such as cytokine release syndrome, immune effector cell-associated neurotoxicity syndrome, and abnormal blood counts, can become serious or life-threatening. Patients and caregivers receive careful instructions to monitor for signs and symptoms of side effects. These conditions are managed by the healthcare provider.
Cytokine release syndrome (CRS)
CRS is caused by a widespread release of molecules called cytokines, which can cause inflammation and can affect the function of various organs. Cytokines may be released by the T cells to which Talvey binds, or by other immune cells in the patient’s body. Signs and symptoms of CRS include fever, low blood pressure, hypoxia (low oxygen levels), fast heartbeat rate, shortness of breath, and chills. CRS typically occurs within a few hours or days after the most recent dose of Talvey in treatment cycle 1. A healthcare provider should be immediately notified if symptoms occur.
Immune effector cell-associated neurotoxicity syndrome (ICANS)
Some of the cytokines released during CRS can result in disruption of the blood–brain barrier, leading to the development of neurological toxicities. ICANS can occur within hours, days, or weeks of treatment with Talvey. Symptoms of ICANS include shaking, lethargy, seizures, confusion, disorientation, and difficulty with speech or handwriting. A healthcare provider should be immediately notified if symptoms occur.
For a more complete list of possible side effects, see the full prescribing information.
Manufacturer
Janssen Biotech, Inc.
Approval
FDA and EMA
Links to drug websites
Last updated: August 17, 2023
How is this drug name pronounced?
Talquetamab: Tal-KAY-tah-mab Talvey: TAL-vee
What cancer(s) does this drug treat?
Multiple Myeloma
Talvey is approved for:
- Patients with multiple myeloma who have received at least three prior treatments for their disease, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody (e.g., [daratumumab [Darzalex]](https://ucir.org/immunotherapy-drugs/daratumumab) or [isatuximab-irfc [Sarclisa]](https://ucir.org/immunotherapy-drugs/isatuximab-irfc)), but whose cancer has progressed on their most recent treatment.
Limitations of use
Limitations: Talvey should only be administered in a treatment center and by adequately trained healthcare professionals who can ensure proper monitoring and immediate management of side effects associated with the drug.
Age: The safety and efficacy of Talvey in patients under 18 years of age have not been established.
Pregnancy/Breastfeeding: Talvey is not recommended for use during pregnancy due to potential for harm to the fetus. Patients who can become pregnant are advised to use contraception during treatment with Talvey and for 3 months after the last dose of Talvey. The risks associated with Talvey during breastfeeding are not known and cannot be ruled out. Due to the potential for adverse reactions in the breastfed child, patients are advised not to breastfeed during treatment with Talvey and for 3 months after the last dose of Talvey.
Live virus vaccination: Vaccination with live virus vaccines (e.g., chickenpox, measles/mumps/rubella [MMR]) is not recommended for at least 4 weeks prior to the start of Talvey treatment and until at least 4 weeks after Talvey treatment.
Driving/Use of Machines: Talvey impacts patients’ ability to drive or use machines due to the increased risk for reduced levels of consciousness. Patients should avoid driving or operating machines during the step-up phase and for at least 48 hours after the completion of the step-up phase. Patients should also avoid driving if they at any point experience new neurological symptoms and should continue to do so until symptoms resolve.
Interactions with other drugs: Dose adjustments may become necessary for patients treated with certain CYP substrates at the start of treatment with Talvey.
What type of immunotherapy is this?
How does this drug work?
Targets:
- GPRC5D on multiple myeloma cells
- CD3 receptor on T cells
Talvey is a “bispecific antibody”. Antibodies are molecules that can bind to a particular target molecule. Bispecific antibodies are artificially made in the laboratory and can bind to two different targets at the same time. Talvey binds to:
- a molecule called GPRC5D on the surface of multiple myeloma cells. While GPRC5D is also present on some healthy cells, including plasma cells and epithelial cells in certain tissues, it is present at much higher quantities on the surface of cancer cells than normal healthy cells.
- a molecule called CD3 on the surface of T cells – the primary immune cells involved in killing cancer cells. CD3 is part of the T cell receptor, which is critical to the function of the T cell and is involved in stimulating the T cell to become active.
Because Talvey can bind to one molecule on multiple myeloma cells and another on T cells at the same time, it acts as a bridge and keeps the T cell in close contact with the cancer cell. By binding CD3 on the T cell, Talvey also stimulates the T cell to become activated and kill the cell it is bound to. Bispecific antibodies that direct T cells to kill cancer cells by binding to both cells at the same time are known as bispecific T cell engagers (BiTEs).

How is the drug given to the patient?
Talvey is given as an injection just below the skin (subcutaneous injection) in the lower part of the abdomen or the thigh. Patients receive Talvey on either a weekly or biweekly (every 2 week) basis. For patients on a weekly schedule, a small dose of Talvey is given on day 1, followed by larger dose on day 3, and a full-sized dose on day 5. For patients on a biweekly schedule, a small dose of Talvey is given on day 1, followed by larger dose on day 3, another larger dose on day 5, and a full-sized dose on day 7. This “step-up” dosing helps to reduce the risk of a reaction to Talvey. Additionally, during step-up dosing, patients receive several medications 1 to 3 hours prior to each dose of Talvey to help reduce the risk of cytokine release syndrome:
- A corticosteroid (dexamethasone)
- An antihistamine (diphenhydramine)
- A painkiller and fever reducer (acetaminophen)
Patients may continue to receive pre-treatment medications prior to full-sized doses of Talvey if dosing is delayed or if certain adverse reactions occur. During step-up dosing, each dose of Talvey requires a 48-hour hospital stay to monitor for potentially serious reactions to treatment.

What are the observed clinical results?
It is important to keep in mind that each patient’s outcome is individual and may be different from the results found in the clinical studies.
Multiple Myeloma
In a clinical study, 143 patients with multiple myeloma who had received at least three prior treatments for their disease, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody, but whose cancer either did not respond to treatment or had since come back, were treated with Talvey on a weekly schedule at a lower dose. The median time to first response was one month. At a median follow-up of 19 months:
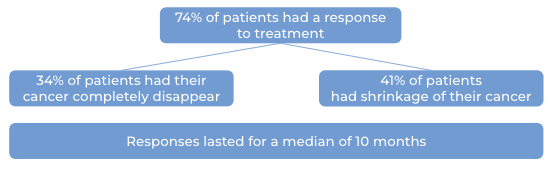
Note: Rounding percentages after the decimal can cause inconsistencies between the total and the sum of the parts.
Further, 145 patients received biweekly (once every two weeks) dosing with Talvey at a higher dose. The median time to first response was one month. At a median follow-up of 13 months:
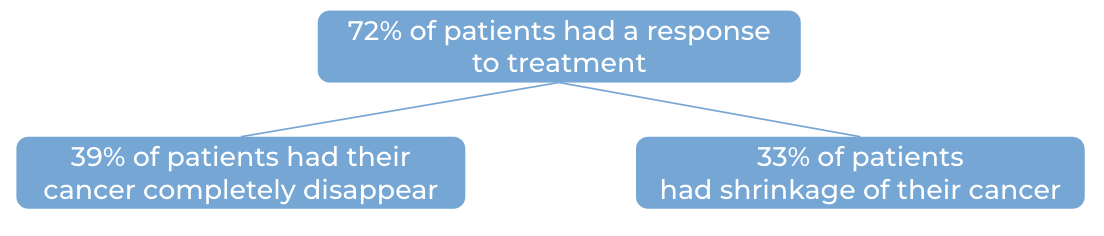
Note: Rounding percentages after the decimal can cause inconsistencies between the total and the sum of the parts.
What are the potential side effects?
The most common side effects of Talvey include cytokine release syndrome, changes in sense of taste, low production of antibodies, nail disorder, pain in the joints, bones, or muscles, low red blood cells (anaemia), fatigue, weight loss, skin problems, rash, dry mouth, low immune cell counts (neutropenia), fever, dryness/itchiness/flaking of the skin, low platelet counts, upper respiratory tract infection, low white blood cell counts (lymphopenia), trouble swallowing, diarrhea, severe itchiness, cough, pain, decreased appetite, and headache.
Some side effects, such as cytokine release syndrome, fever, immune cell-associated neurotoxicity syndrome (ICANS), infections, and abnormal blood counts, can become serious or life-threatening. Patients and caregivers receive careful instructions to monitor for signs and symptoms of side effects. These conditions are managed by the healthcare provider.
Cytokine release syndrome (CRS)
CRS is caused by a widespread release of molecules called cytokines, which can cause inflammation and can affect the function of various organs. Cytokines may be released by the T cells to which Talvey binds, or by other immune cells in the patient’s body. Signs and symptoms of CRS include fever, low blood pressure, hypoxia (low oxygen levels), fast heartbeat rate, shortness of breath, and chills. CRS typically occurs within a few hours or days after the most recent dose of Talvey in treatment cycle 1. A healthcare provider should be immediately notified if symptoms occur.
Immune effector cell-associated neurotoxicity syndrome (ICANS)
Some of the cytokines released during CRS can result in disruption of the blood–brain barrier, leading to the development of neurological toxicities. ICANS can occur within hours, days, or weeks of treatment with Talvey. Symptoms of ICANS include shaking, lethargy, seizures, confusion, disorientation, and difficulty with speech or handwriting. ICANS typically occurs within a few hours or days after the most recent dose of Talvey in treatment cycle 1. A healthcare provider should be immediately notified if symptoms occur.
Infections
Talvey can alter the immune system by eliminating healthy plasma B cells, leaving patients more vulnerable to infections. Severe, life-threatening, or fatal infections have been reported in patients receiving Talvey.
For a more complete list of possible side effects, see the full prescribing information.
Manufacturer
Janssen
Approval
FDA and EMA
Links to drug websites
Last updated: October 10, 2025








