How is this drug name pronounced?
Toripalimab-tpzi: toh-ree-PAH-lih-mab
Loqtorzi: lock-TOHR-zee
What cancer(s) does this drug treat?
Nasopharyngeal Cancer
Loqtorzi is approved for:
- Patients with nasopharyngeal cancer that has spread (metastatic) or has returned (is recurrent) and is locally advanced. In such cases, patients are treated with Loqtorzi in combination with cisplatin and gemcitabine chemotherapies
- Patients with nasopharyngeal cancer that has come back (recurrent) and cannot be removed by surgery, or that has spread (metastasized) or gotten worse on or after treatment with a platinum-containing chemotherapy. In such cases, patients are treated with Loqtorzi alone.
Limitations of use:
Age: The safety and efficacy of Loqtorzi in patients under 18 years of age have not been established.
Fertility/Pregnancy/Breastfeeding: Loqtorzi can cause harm or death to a fetus and is not recommended for use during pregnancy. Pregnancy should be prevented during treatment with Loqtorzi and for at least four months after the last dose of Loqtorzi. Due to the potential for serious adverse reactions in the breastfed child, patients are advised not to breastfeed during treatment and for at least four months after the last dose of Loqtorzi.
Complications of stem cell transplant: Serious and life-threatening transplant-related complications can occur in patients who receive a stem cell transplant from a donor before or after being treated with Loqtorzi.
Solid organ transplant rejection: Treatment with Loqtorzi can increase the risk of rejection in patients who have received an organ transplant.
What type of immunotherapy is this?
- PD-1 blockade
How does this drug work?
- Target: PD-1
Loqtorzi is an antibody that attaches to a molecule called PD-1, which is present on the surface of T cells. In healthy T cells, PD-1 acts as a brake that prevents the cells from creating an out-of-control immune response. However, in tumors, PD-1 can make T cells inactive and can prevent them from doing their job of killing the cancer cells. This happens because cancer cells or other cells within the tumor have an increased concentration of PD-L1 and PD-L2 molecules on their cell surface, which attach to PD-1. When PD-L1 and PD-L2 interact with the PD-1 receptor on the T cell, the PD-1 sends a signal to the T cell to become inactive. Loqtorzi binds to the PD-1 on the T cells in a way that blocks PD-L1 and PD-L2 from interacting with the PD-1 receptor. This blockade on PD-1 allows the T cells to activate and to attack and kill cancer cells.
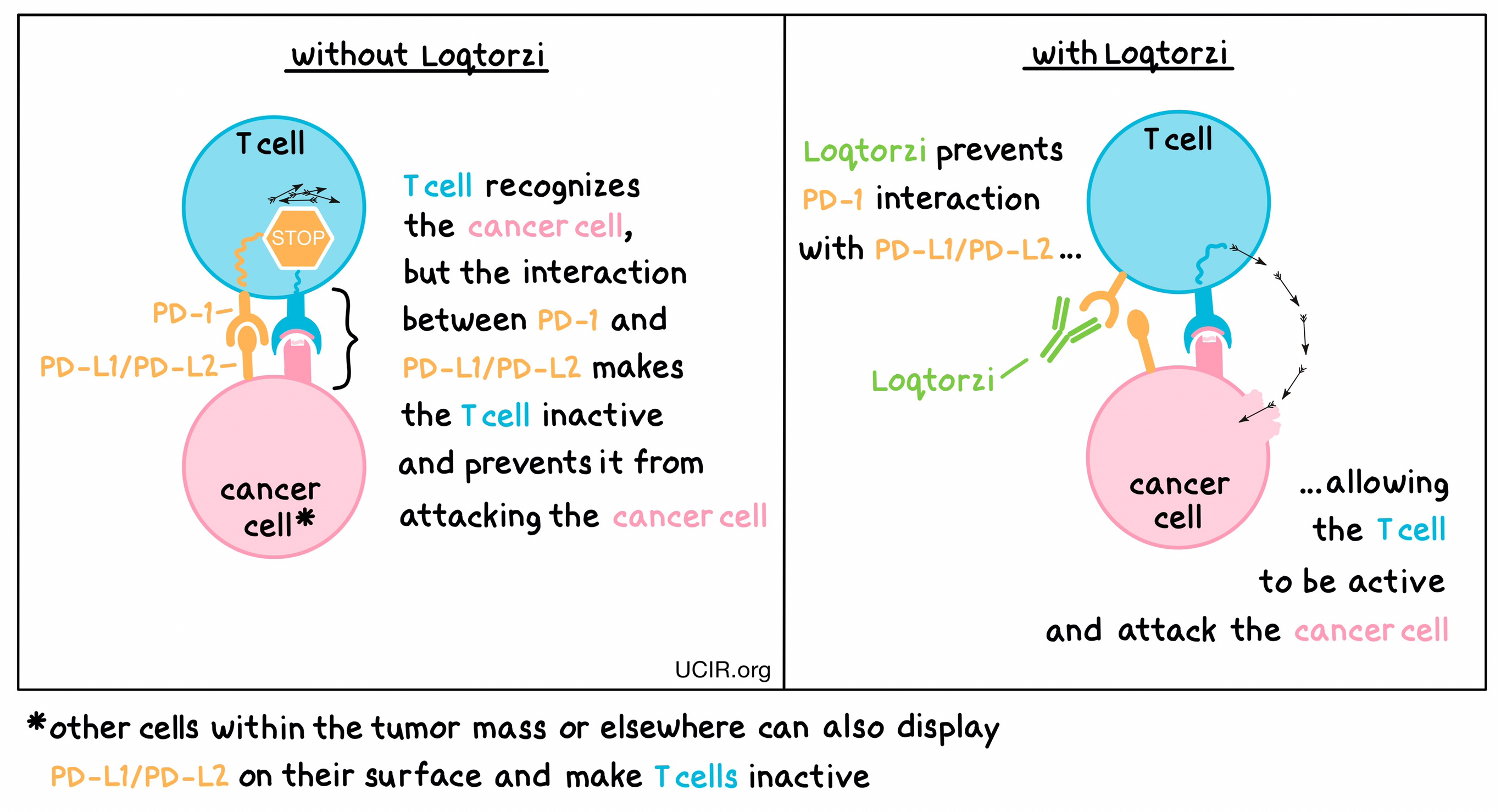
How is this drug given to patients?
Loqtorzi is administered via a tube into a vein (intravenous infusion or i.v.). For the first infusion, Loqtorzi is given over the course of 60 minutes; if there are no negative reactions to the first infusion, subsequent infusions can be given over the course of 30 minutes. When Loqtorzi is being used alone, it is administered every two weeks until the disease progresses. When used in combination with cisplatin and gemcitabine, Loqtorzi is administered every three weeks until the disease progresses, or for up to 24 months.
What are the observed clinical results?
For:
Nasopharyngeal Cancer (metastatic or recurrent)
Nasopharyngeal Cancer (previously treated with a platinum-based chemotherapy)
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Nasopharyngeal cancer (metastatic or recurrent)
In a clinical trial, 289 patients with nasopharyngeal cancer that had spread (metastatic) or had returned (is recurrent) and was locally advanced were treated with Loqtorzi in combination with cisplatin and gemcitabine chemotherapy or with placebo and cisplatin/gemcitabine chemotherapy.
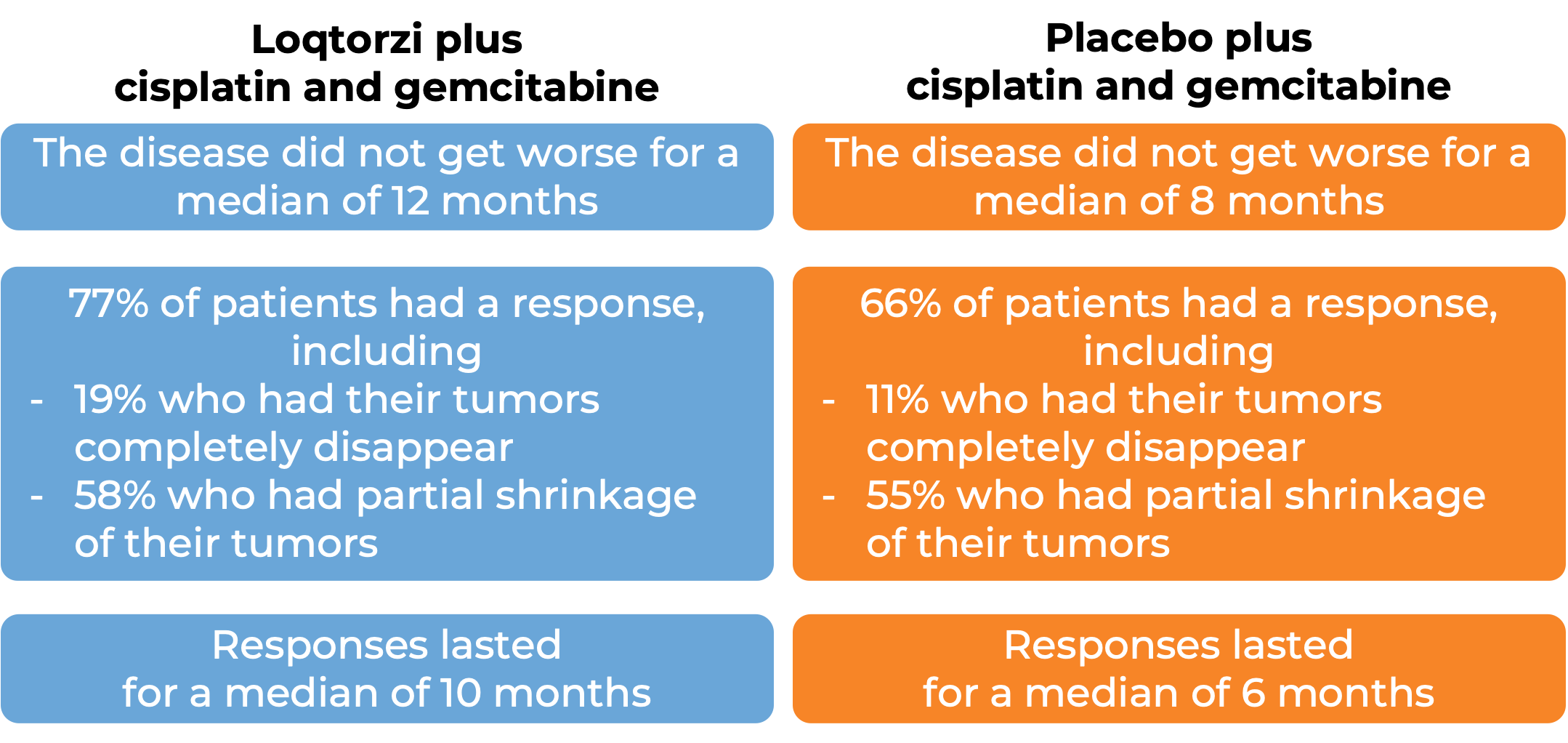
(For the definition of “median”, click HERE.)
Nasopharyngeal Cancer (previously treated with a platinum-based chemotherapy)
In a clinical trial, 172 patients with nasopharyngeal cancer that had come back (recurrent) and could not be removed by surgery, or that had spread (metastasized), or gotten worse on or after treatment with a platinum-containing chemotherapy were treated with Loqtorzi.
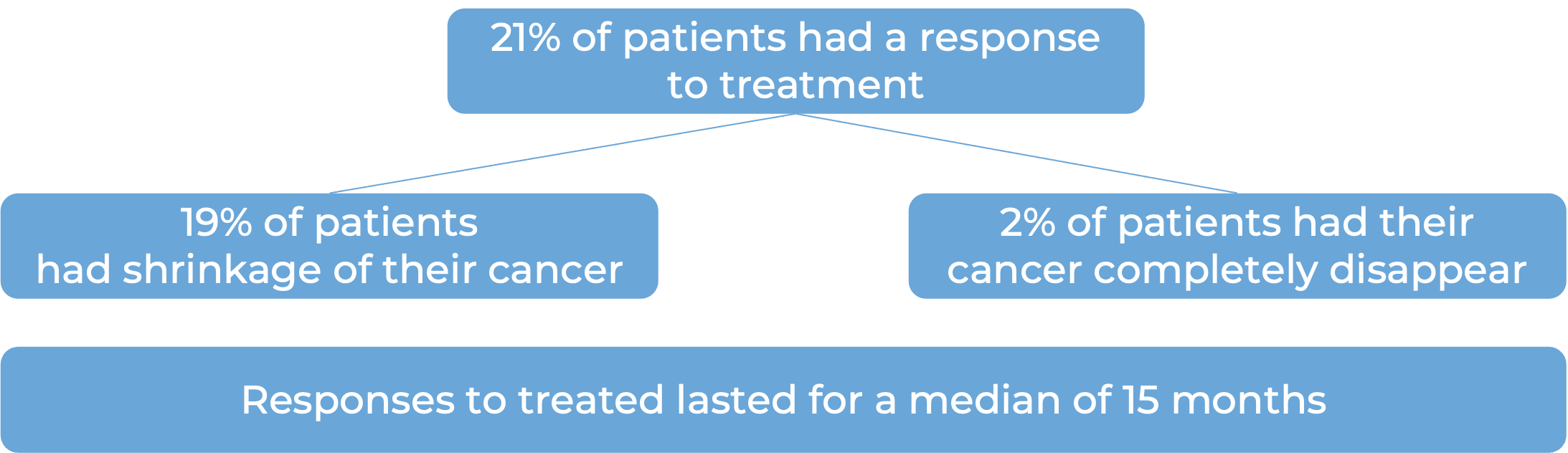
(For the definition of “median”, click HERE.)
What are the side effects?
The most common side effects of Loqtorzi alone are fatigue, hypothyroidism and pain in the bones and muscles. The most common side effects for Loqtorzi used in combination with cisplatin and gemcitabine are nausea, vomiting, decreased appetite, constipation, underactive thyroid gland, rash, fever, diarrhea, numbness or tingling in the hands or feet, cough, pain in the bones and muscles, upper respiratory infection, trouble falling and/or staying asleep, dizziness, and general discomfort.
Loqtorzi can cause a patient’s immune cells to attack healthy cells throughout the body. Because of this, Loqtorzi can cause side effects that can become serious or life-threatening, and may lead to death. Some of the serious side effects related to Loqtorzi include inflammation of the lungs, colon, liver, or kidneys. Additionally, problems can arise with hormone glands. Loqtorzi may cause Type 1 diabetes. Serious skin problems, severe infections, and reactions related to the infusion may also occur.
Loqtorzi can cause serious and life-threatening complications, including graft-versus-host disease in patients who have received a stem cell or solid organ transplant before or after being treated with Loqtorzi. These complications may arise even if patients have been treated with other types of therapy in between administration of Loqtorzi and stem cell transplant.
Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects. For a more complete list of possible side effects, see the full prescribing information.
Additional Information
Manufacturer:
Coherus BioSciences, Inc.
Approval:
- FDA and EMA
LInks to drug websites:
Last updated on January 2, 2024


