How is this drug name pronounced?
ziv-Aflibercept: ziv-a-FLIH-ber-sept
Zaltrap: ZAL-trap
What cancer(s) does this drug treat?
Zaltrap is approved for:
Advanced colorectal cancer
- Patients with advanced colon or rectal cancer that has spread to other parts of the body, and who have received therapy containing oxaliplatin (Eloxatin), and it either did not work or stopped working. In such cases, Zaltrap is used in combination with fluorouracil, leucovorin, and irinotecan (Onivyde or Camptosar).
Limitations of use:
Age: The safety and efficacy of Zaltrap in patients under 18 years of age have not been established.
Fertility/Pregnancy/Breastfeeding: Zaltrap can impair fertility in women and in men. Zaltrap can cause harm to a fetus, and is not recommended for use during pregnancy. Pregnancy should be prevented for at least three months after the last dose of Zaltrap. The risks associated with Zaltrap during breastfeeding are not known and cannot be ruled out. Due to the potential for serious adverse reactions in the breastfed child, women are advised not to breastfeed during treatment and for at least one month after the last dose of Zaltrap.
Impaired wound healing: Zaltrap should not be administered at least 4 weeks before and after major surgery, and until wounds are healed.
What type of immunotherapy is this?
- Angiogenesis inhibitor
How does this drug work?
- Targets: vascular endothelial growth factor (VEGF) and placental growth factor (PlGF)
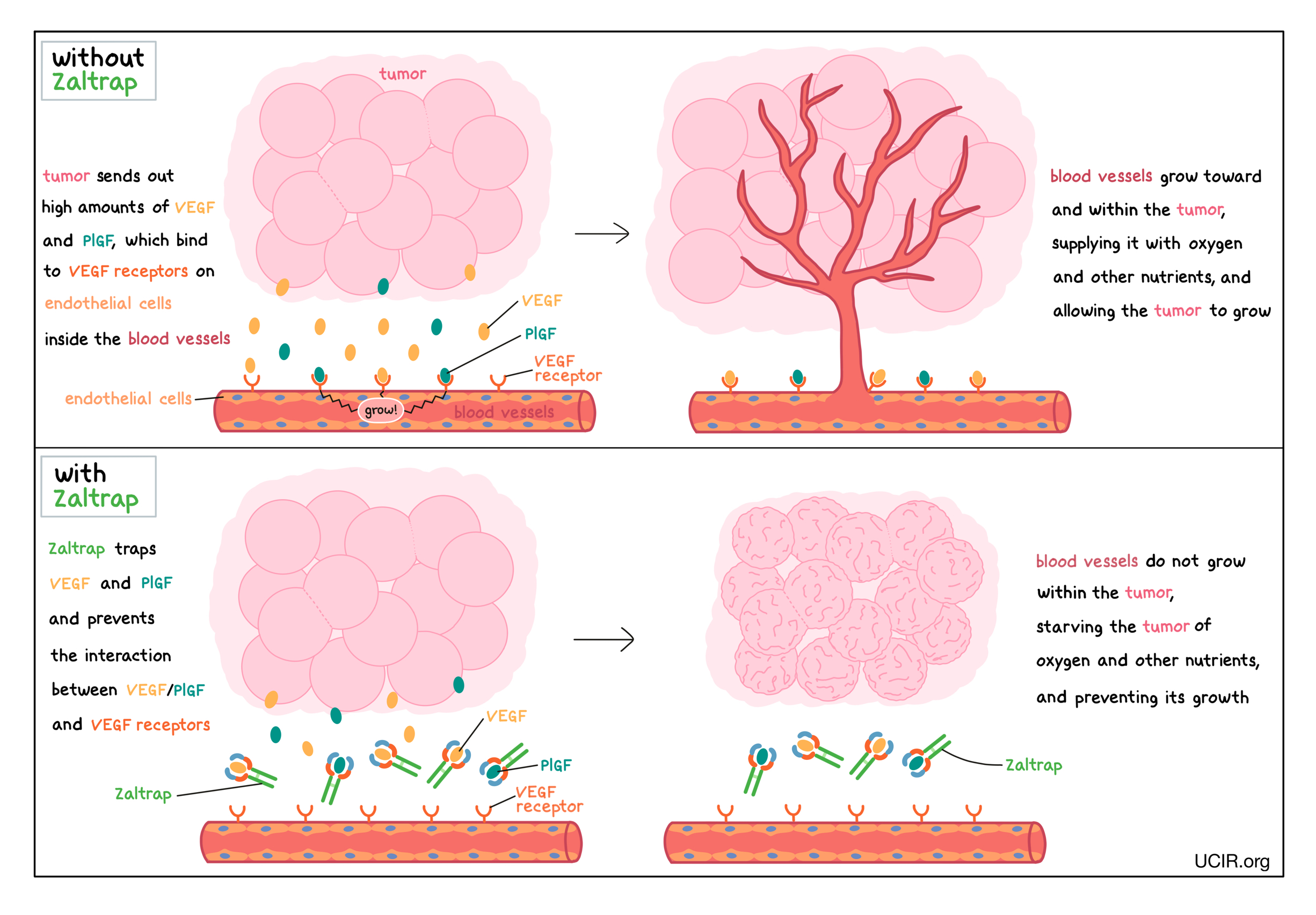
Zaltrap is a protein made in a laboratory that can attach to either of two molecules – vascular endothelial growth factor (VEGF) or placental growth factor (PlGF). VEGF and PlGF stimulate the formation of new blood vessels and promote growth of preexisting blood vessels – a process called angiogenesis.
In a healthy body, VEGF is produced by many different types of cells and helps to create new blood vessels during embryonic development, normal growth of tissues, and wound healing. PlGF is produced by the placenta during pregnancy, and is also produced in a healthy body at low levels in other organs, including the heart, lungs, thyroid, and muscles. VEGF and PlGF bind to molecules called VEGF receptors that are present on endothelial cells, which can be found lining the insides of blood vessels. The binding of VEGF or PlGF to VEGF receptors leads the endothelial cells to multiply, migrate to the source of the VEGF and PlGF molecules, and form new blood vessels. Tumors make higher than normal amounts of VEGF and PlGF because the formation of new blood vessels within the tumor is critical for the tumor to grow (by providing the tumor with access to oxygen and other nutrients) and to spread to other parts of the body (by providing a route for cancer cells to enter blood circulation and move to other locations).
Zaltrap works by trapping the VEGF and PlGF molecules that float within tissues or blood vessels in a way that inhibits the interaction between VEGF/PlGF and the VEGF receptors on endothelial cells. This helps prevent the formation of new blood vessels within the tumor, thus starving the tumor of oxygen and other nutrients, and preventing its growth.
How is this drug given to the patient?
Zaltrap is administered via a tube into a vein (intravenous infusion, or I.V.) over one hour every two weeks, and does not require a hospital stay.
Zaltrap should not be administered for at least 4 weeks prior to elective surgery, as well as for at least 4 weeks after major surgery, and only after the surgical wound is fully healed.
What are the observed clinical results?
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Advanced colorectal cancer
In a clinical trial, 1226 patients with advanced colon or rectal cancer that had spread to other parts of the body, and who had received therapy containing oxaliplatin (Eloxatin), and it either did not work or stopped working, were treated with either:
- Zaltrap plus leucovorin, irinotecan (Onivyde or Camptosar), and fluorouracil, OR
- Placebo plus leucovorin, irinotecan, and fluorouracil.
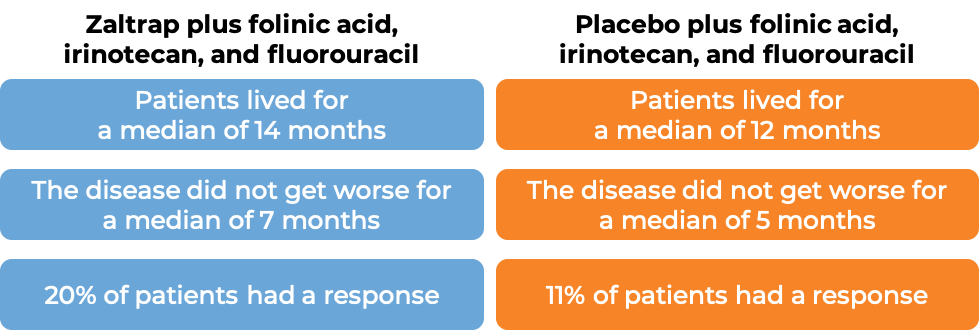
(For the definition of “median”, click HERE.)
What are the side effects?
The most common side effects of Zaltrap include fatigue, weakness, diarrhea, painful sores in the mouth, decreased appetite, stomach pain, headache, nose bleed, high blood pressure, hoarseness, low white blood cell count, low platelet count, increase in liver enzymes, changes in kidney function, and too much protein in the urine.
Zaltrap can cause side effects that can become serious or life-threatening, and may lead to death. Some of the serious side effects related to Zaltrap include development of a hole in the stomach or intestine, formation of an abnormal connection between two body parts (a fistula), blood clots, serious bleeding, having wounds that do not heal, severe diarrhea and dehydration, bone breakdown in the jaw, and severe high blood pressure. Additionally, problems can arise with the kidneys, the heart, and the brain. Zaltrap may cause low white blood cell count, which could lead to serious infections. Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects.
For a more complete list of possible side effects, see the full prescribing information.
Additional information
Manufacturer
Sanofi-Aventis
Approval
FDA and EMA
Links to drug websites
Last update on August 6, 2025


