Can the bacteria in your gut help your immune system fight cancer?
2019-02-21
The human gut is filled with microbes, and these tiny organisms can have a big impact – affecting a broad range of bodily functions from metabolism, to mood, to the immune system. In a recent study, lead researchers Takeshi Tanoue and Satoru Morita and others, working under the guidance of Kenya Honda from Keio University in Tokyo, Japan, took a closer look at how the microbes in the gut, together called the gut microbiome, affect the immune system, and whether they could manipulate the microbiome to improve immune responses to infections or cancer.
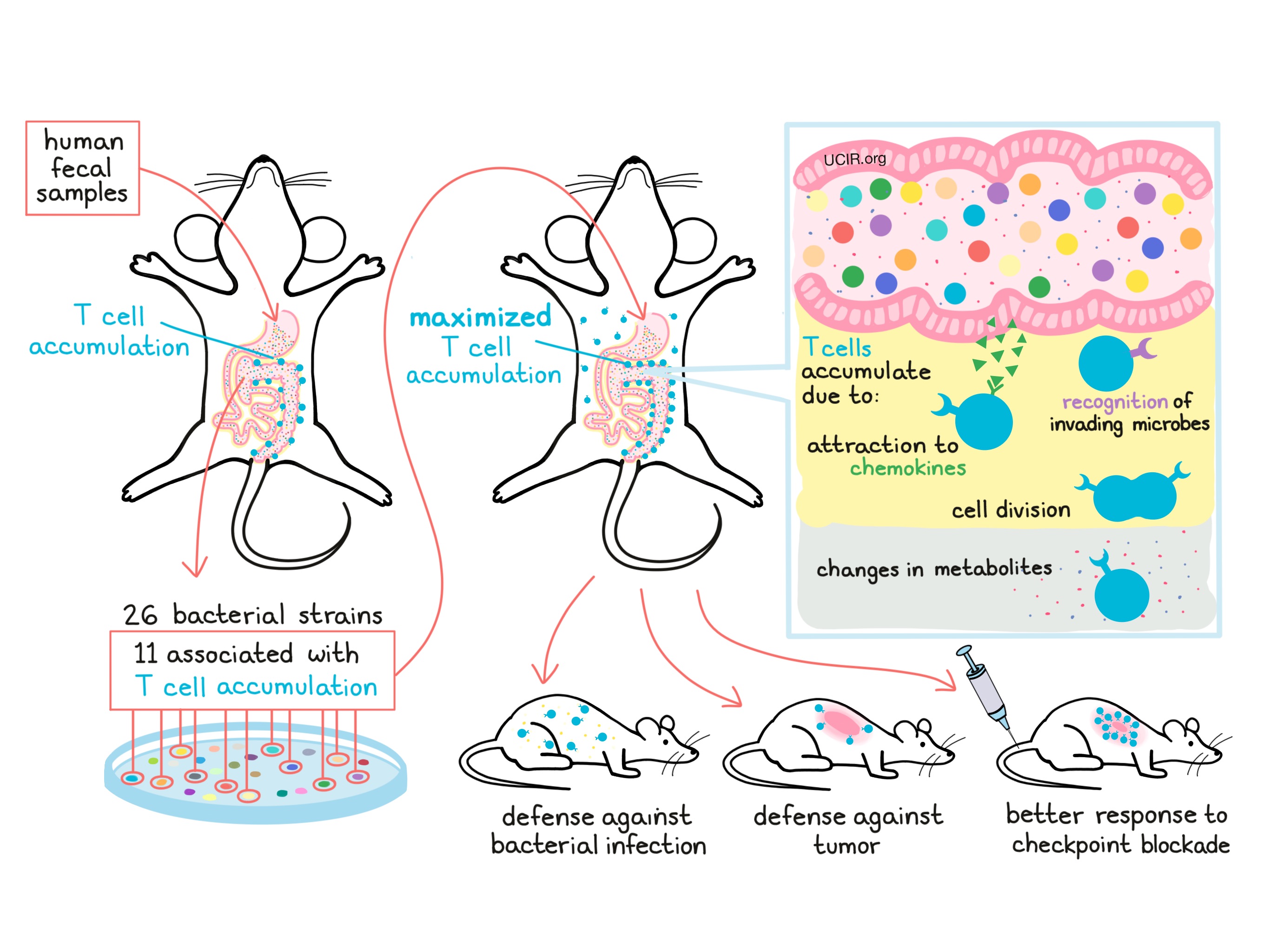
Research began when Tanoue and Morita noticed an interesting phenomenon. They found that mice with a normal gut microbiome had far more T cells (the front-line fighters of the immune system) built up just behind the lining of the gut than either mice treated with microbe-killing antibiotics or “germ-free” mice which are bred to have no microbes in them at all. When the researchers transferred a microbiome into germ-free mice, T cells began to accumulate just outside the gut, (as they did in normal mice), confirming that something about the microbiome was drawing the attention of the immune system.
With the goal of using the microbiome to enhance the immune system, the researchers began determining which specific microbes in the gut had the ability to promote T cell accumulation. To make their research relevant to potential patients, they used stool samples – or in other words, poo – from six healthy human volunteers to populate the guts of mice. The mouse with the highest accumulation of T cells around its gut was selected for further study. From the gut microbiome of this mouse, the researchers were able to identify 26 unique strains of bacteria, 11 of which were associated with the accumulation of T cells. When a mix of just these 11 strains was given to a new mouse, it induced the strongest T cell accumulation seen yet, even causing accumulation in distant organs. Furthermore, the researchers found that the 11 strains of microbes worked together as a team, and that taking any one strain away made the group less effective.
Wondering how this 11-strain team actually worked to cause T cell accumulation around the gut, the researchers took a closer look at the mechanisms and identified several possible contributing factors. First, they found that the microbes cause nearby human cells that make up the lining of the gut to produce molecules called chemokines that attract T cells to the site. Next, they found that many T cells were actively dividing, which would further add to the accumulation. Some of the nearby T cells actually recognized the microbes as foreign invaders, meaning that a direct activation of the immune system by the microbiome might be drawing in T cells. Finally, the researchers found that some unique metabolites (byproducts produced by the gut microbes) could be found circulating throughout the blood of the mice, which may be why T cells also accumulated in distant organs.
The researchers then wondered whether having those T cells around the gut might mean that the immune system was better armed when faced with a threat. To test this, the researchers treated mice with the 11-strain mix of gut microbes and then infected them with Listeria bacteria to cause symptoms of food poisoning. Mice with the specialized microbiome cleared the infection much faster and with less damage compared to mice with no microbiome or even those with a typical microbiome. Similarly, when mice with the specialized microbiome were injected with cancerous cells, their immune systems did a better job controlling and slowing down tumor growth. When tumor-bearing mice were also treated with checkpoint blockade therapies used to treat certain human cancers, treatment was most effective in mice with the 11-microbe mix in their guts.
With such favorable results in mice, the researchers wondered whether the 11 gut microbes they identified could also be used to enhance immunity in humans. Looking at large sets of data for human gut microbiomes, they found that the 11 strains were generally rare within the human microbiome. Because of their underrepresentation, it is possible that introducing these strains of T cell-attracting bacteria into patients might help them to boost their immunity and response to immunotherapy. The actual effect of introducing these strains into humans, however, is not yet known, and further research will be needed to determine whether the promising results seen here in mice could translate to the clinic.
For a more in-depth analysis of this study, visit ACIR.org.
by Lauren Hitchings



