How is this drug name pronounced?
Axicabtagene ciloleucel: AK-see-KAB-tuh-jeen sy-loh-LOO-sel
Yescarta: yes-KAR-tuh
What cancer(s) does this drug treat?
Yescarta is approved for:
Large B-cell non-Hodgkin lymphoma
Follicular lymphoma
Large B-cell non-Hodgkin lymphoma
Yescarta is approved for:
- Adults with large B-cell lymphoma whose cancer either did not respond (refractory), or responded to treatment with chemoimmunotherapy, but then came back (relapsed) within 12 months of treatment.
- Adults with large B-cell non-Hodgkin lymphoma (including diffuse large B-cell lymphoma (DLBCL), primary mediastinal large B-cell lymphoma (PMBCL), high grade B-cell lymphoma, and DLBCL arising from follicular lymphoma) whose cancer either did not respond (refractory) to treatment, or responded to two or more previous treatments, but then came back (relapsed).
Advanced follicular lymphoma
Yescarta is approved for:
- Adults with follicular lymphoma, whose cancer did not respond (refractory) to treatment, or responded to two or more previous treatments, but then came back (relapsed).
Limitations of use
REMS Program: Due to the potential for serious side effects, Yescarta is currently only available through a Risk Evaluation and Mitigation Strategy (REMS) program. Healthcare facilities that plan to treat patients with Yescarta must first qualify for the program.
Central Nervous System Lymphoma: Yescarta has not been approved for patients with primary central nervous system lymphoma.
Age: Yescarta has not been studied in pediatric patients under the age of 18.
Fertility/Pregnancy/Breastfeeding: The effects of Yescarta on fertility are not known. Yescarta is not recommended for patients who are pregnant. The risks associated with Yescarta during breastfeeding are not known, and risk to a breastfed infant cannot be excluded.
Effects on the ability to drive and use machines: Patients are advised to refrain from driving, engaging in potentially dangerous activities, and operating heavy machinery for at least 8 weeks after receiving Yescarta.
What type of immunotherapy is this?
- CAR T cell therapy
How does this drug work?
- Target: CD19
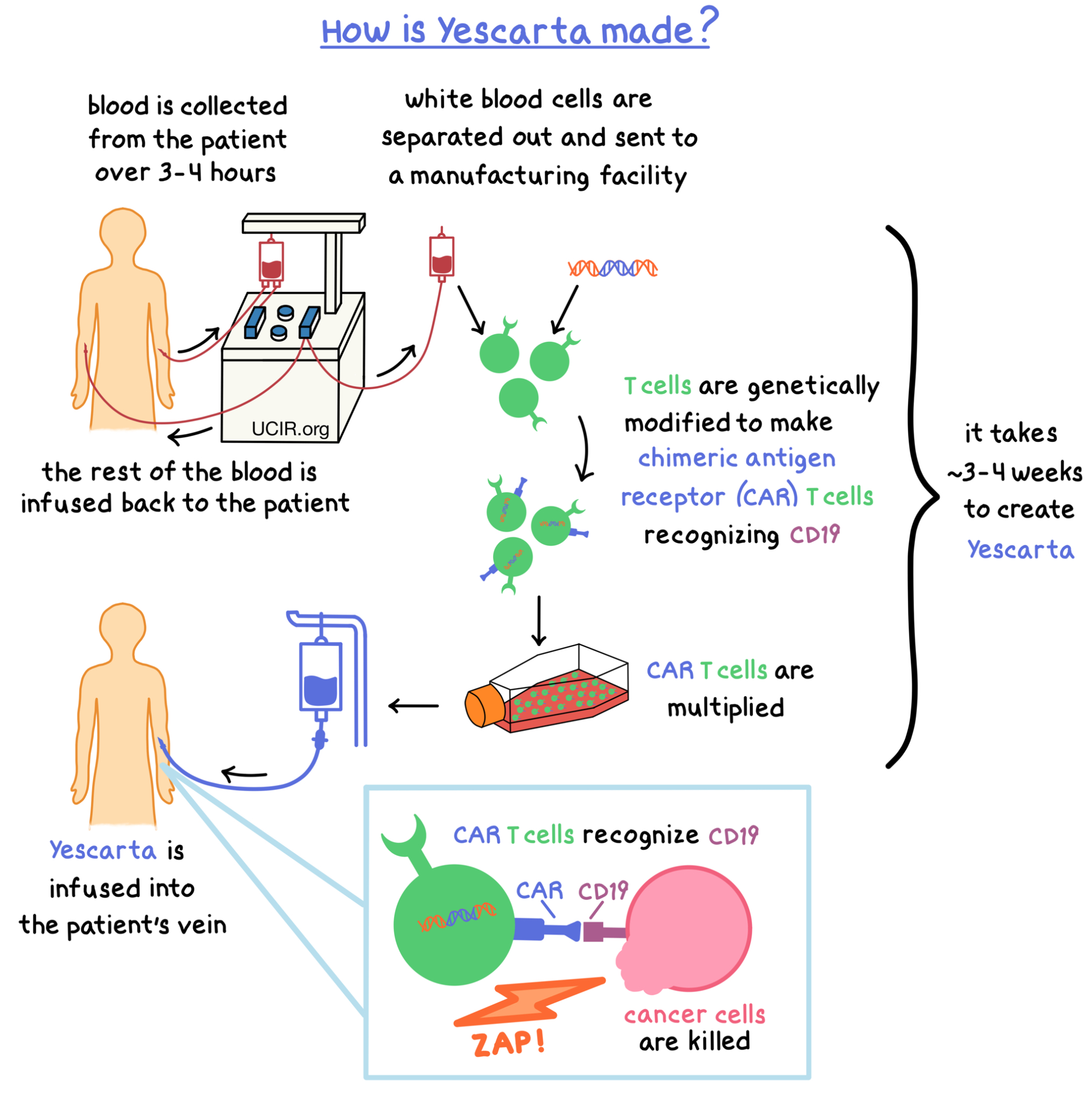
Yescarta is made from the patient’s own T cells (a type of white blood cell). In order to make Yescarta, blood is collected from the patient’s vein – this process usually takes 3 to 4 hours. In a process called leukapheresis, the white blood cells (including T cells) are separated from the collected blood, and the rest of the blood is returned to the patient. The collected white blood cells are sent to a specialized manufacturing facility where the patient’s T cells are genetically modified in such a way that they make a protein on their surface called chimeric antigen receptor (CAR). The modified T cells (“CAR T cells”) are multiplied to create millions of CAR T cells.
The CAR on the surface of the modified T cell can attach to a protein called CD19 on the surface of the cancer cell. When CAR attaches to CD19, T cells recognize the cancer cells and kill them. The entire process to create Yescarta from the collected blood could take approximately 3 to 4 weeks.
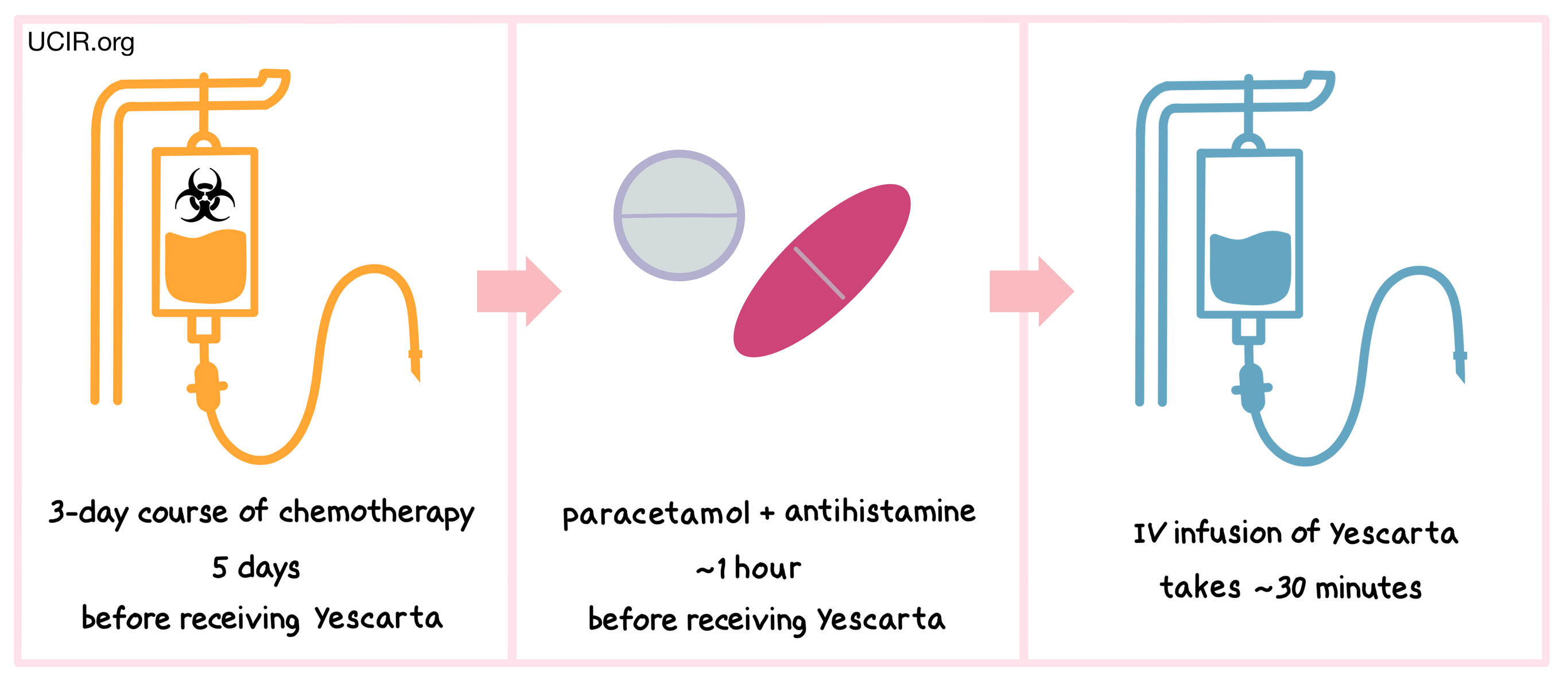
How is this drug given to the patient?
Starting 5 days before receiving Yescarta, patients are treated with a 3-day course of chemotherapy to remove white blood cells. The reduction of white blood cells in the patient gives the CAR T cells in Yescarta enough space to multiply and provides the CAR T cells with enough resources needed to persist in the patient longer. About an hour before receiving Yescarta, patients receive acetaminophen and an antihistamine (and in some cases, corticosteroids) to reduce the chance of reactions to the infusion.
Patients receive Yescarta through an intravenous (IV) infusion (through a tube in the vein). The administration of Yescarta usually takes about 30 minutes.
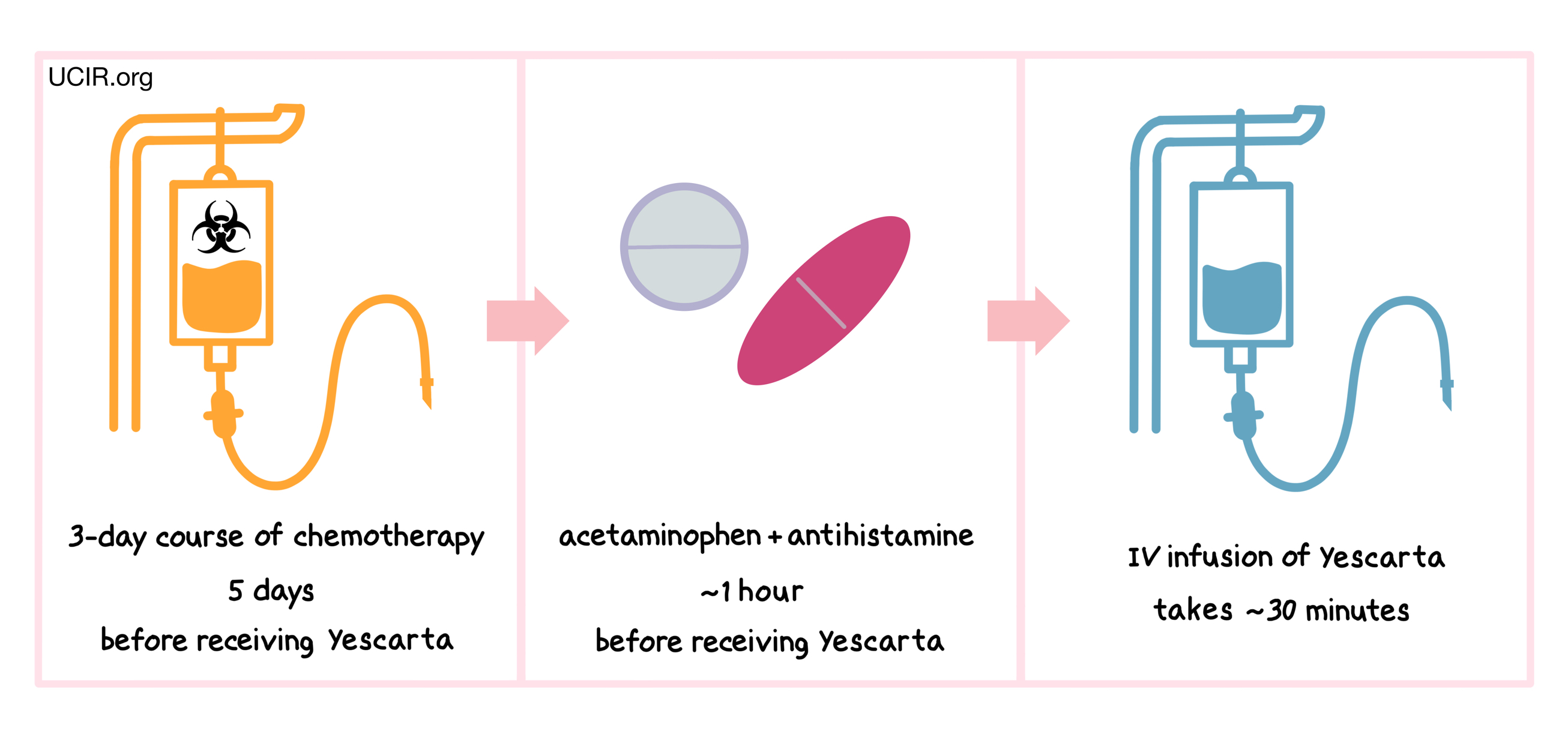
Patients should plan to stay close to the treatment location for at least 4 weeks after receiving Yescarta.
What are the observed clinical results?
For:
Advanced large B-cell non-Hodgkin lymphoma
Advanced follicular lymphoma
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
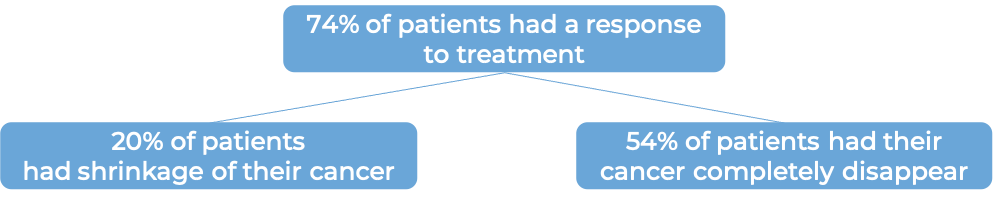
Large B-cell non-Hodgkin lymphoma
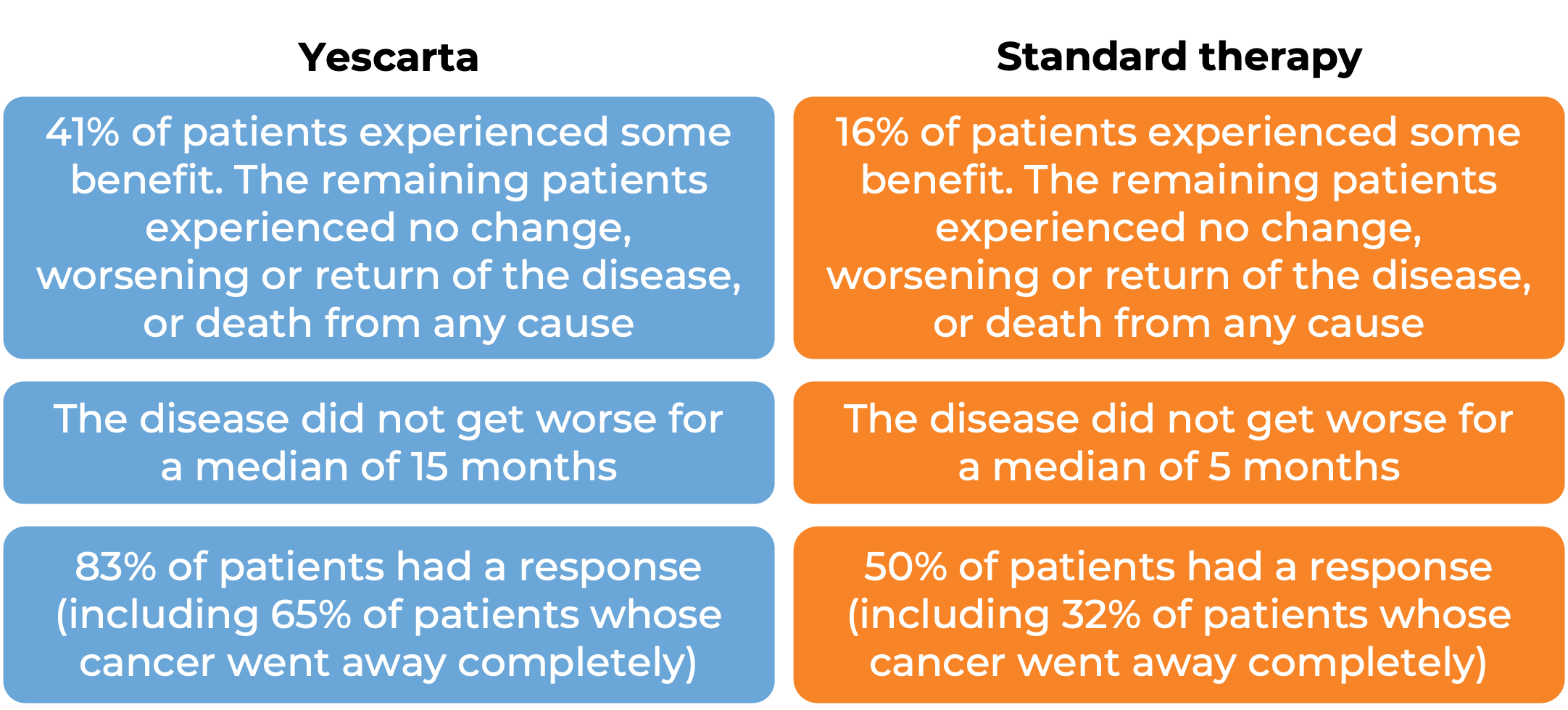
In a clinical trial, 359 patients with large B-cell lymphoma whose cancer either did not respond to treatment with chemoimmunotherapy (anthracycline and rituximab), or came back within 12 months after treatment, were treated with either Yescarta or standard second-line therapy (chemoimmunotherapy and stem cell transplant). At a median follow-up of 25 months:
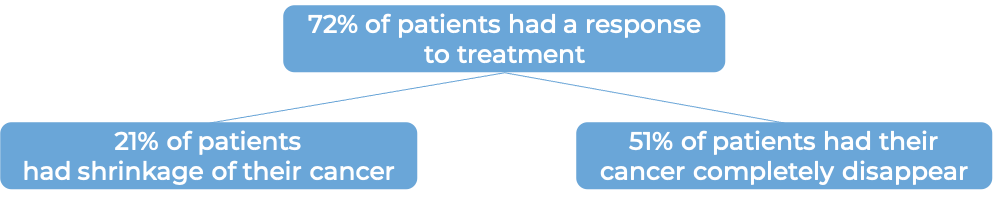
In another clinical trial, 101 patients with advanced, previously treated large B-cell non-Hodgkin lymphoma, were treated with Yescarta. At a median follow-up of 15 months:
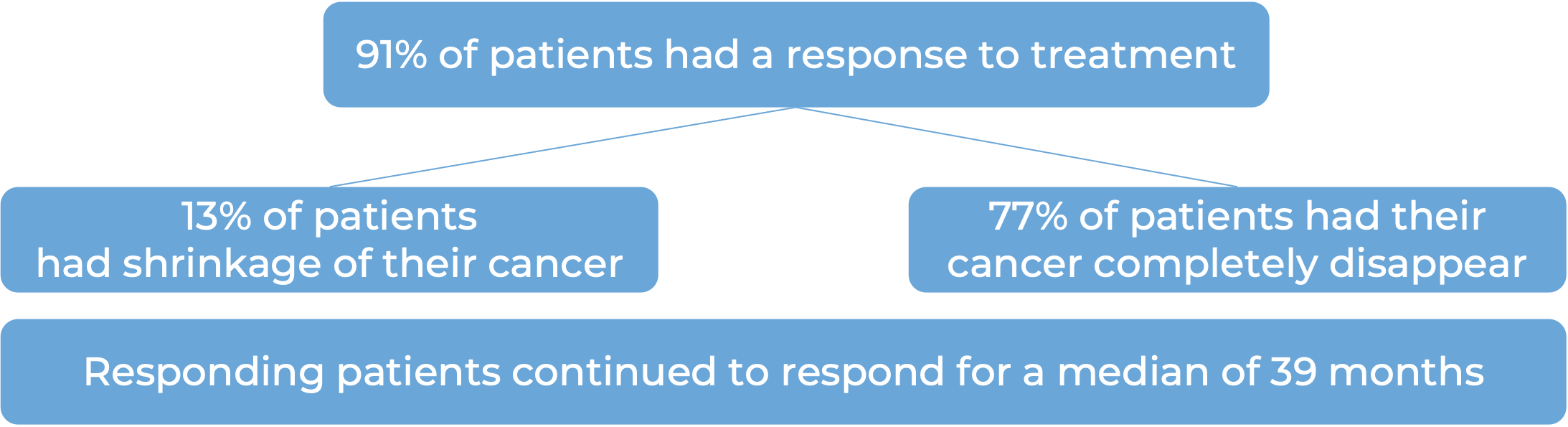
Follicular lymphoma
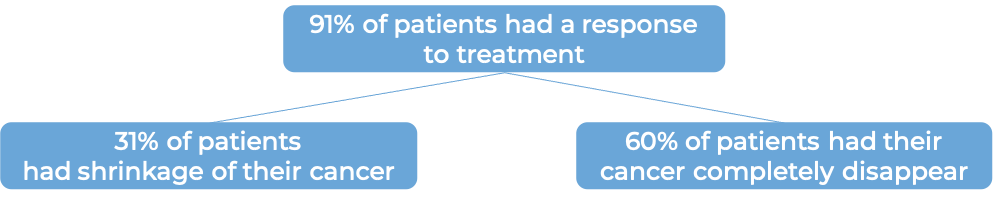
In a clinical trial, 120 adult patients with advanced, previously treated follicular lymphoma, were treated with Yescarta. At a minimum follow-up of 9 months for the first 81 patients:
What are the side effects?
Yescarta can lead to side effects, some of which could be long-lasting. The CAR T cells in Yescarta kill cancer cells that have the molecule CD19 on their surface; however, CD19 is also found on the surface of healthy B cells, which can also be killed by Yescarta. The decrease in healthy B cells leads to low levels of antibodies (immunoglobulins) and an increased risk of serious infections. Other possible side effects include low blood cell counts, low blood pressure, fatigue, fast heartbeat, headache, nausea, diarrhea, muscle pain, altered mental state, and fever, and serious allergic reactions.
Most patients receiving Yescarta experience serious side effects. Some side effects, such as cytokine release syndrome (CRS) and neurological toxicities, may be severe or life-threatening. Patients will be monitored daily by their health care provider for at least 7 days after infusion of Yescarta. Patients and caregivers receive careful instructions to monitor for signs and symptoms related to CRS and neurological toxicities. Both conditions are managed by the health care provider.
Cytokine release syndrome (CRS)
CRS is caused by a widespread release of molecules called cytokines, which are involved in inflammation and can affect the function of various organs. Cytokines may be released by the CAR T cells in Yescarta or by other immune cells in the patient’s body. Signs and symptoms of CRS include high fever, chills, difficulty breathing, fast or irregular heartbeat, severe nausea, vomiting, diarrhea, very low blood pressure, low oxygen level, and dizziness or lightheadedness. CRS typically occurs between 1 and 12 days after Yescarta infusion, and the health care provider should be immediately notified if symptoms occur.
Neurological toxicities
Some of the cytokines released during CRS can result in disruption of the blood-brain barrier, leading to the development of neurological toxicities. Symptoms of neurological toxicities include confusion, altered or decreased consciousness, tremors, seizures, headache, loss of balance, and difficulty speaking and understanding. Neurological toxicities typically occur about 4 to 5 days after Yescarta infusion, but could occur 8 weeks or later after infusion.
For a more complete list of possible side effects, see the full prescribing information.
Additional information
Manufacturer
Kite Pharma, subsidiary of Gilead Sciences
Approval
FDA and EMA
Links to drug websites
Other references
- Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Locke FL, Ghobadi A, et al. The Lancet Oncology (2019).
- Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. Locke FL, Miklos DB, et al. The New England Journal of Medicine (2022)
Last updated January 11, 2023