T Cell Therapy
What is T cell therapy?
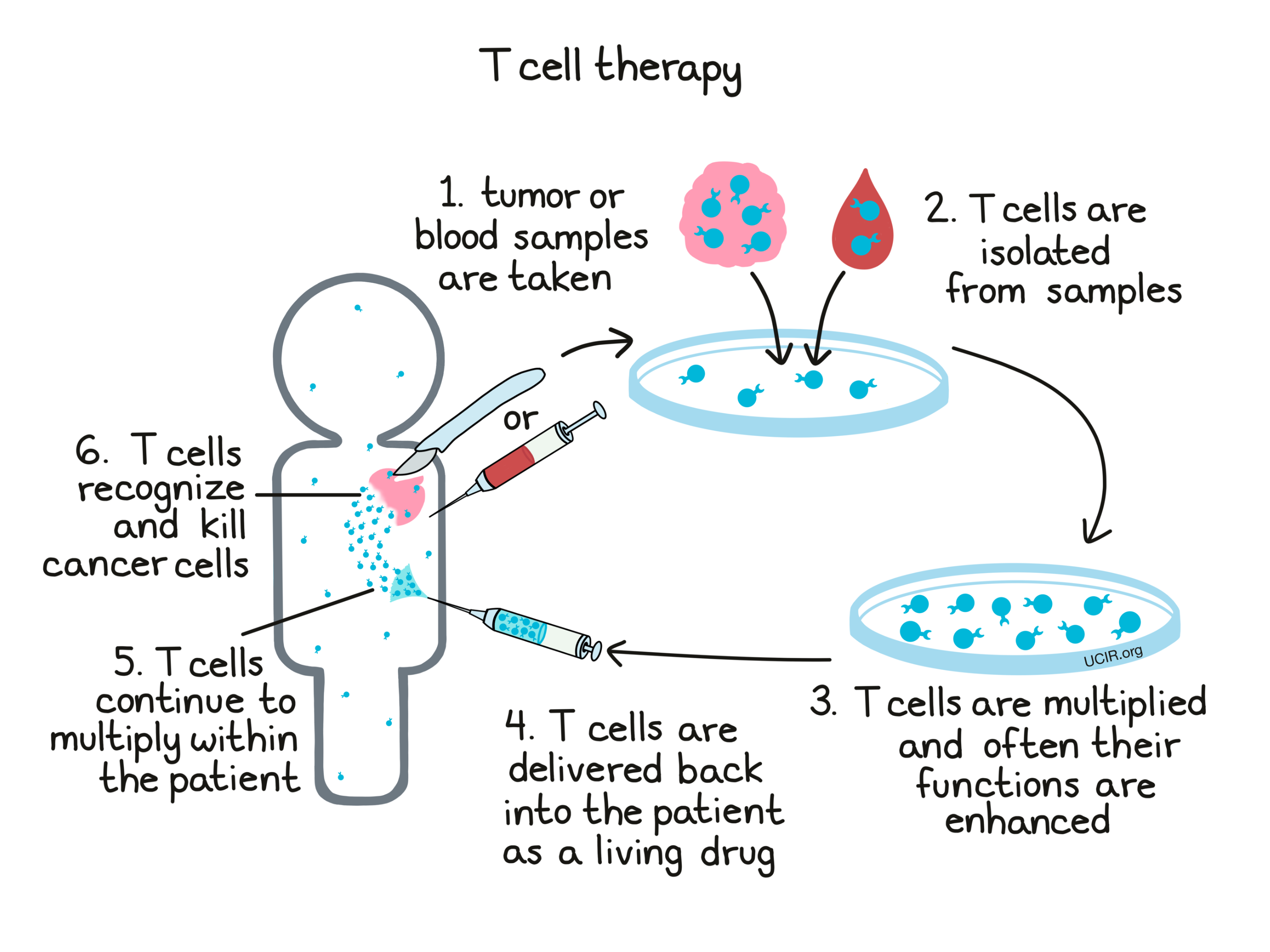
T cell therapy is a potential treatment for cancer that uses the patient's own T cells as a “living drug”. T cells are the cells of the immune system responsible for seeking out and destroying unhealthy cells. These unhealthy cells are typically cells infected with harmful microorganisms such as bacteria or viruses, but some T cells can also naturally recognize and kill cancer cells.
When the immune system is still developing, individual T cells are each “trained” to target one particular molecule (a “target antigen”) on unhealthy or threatening cells – imagine that each T cell is given a mugshot of one particular antigen, and it becomes that T cell’s job to recognize that mugshot and make sure that that particular threat never harms the body. T cells lie in wait in the body, and when a threat is detected, they spring into action, multiplying into a T cell army that hunts down and kills any cells that contain the threatening molecule.
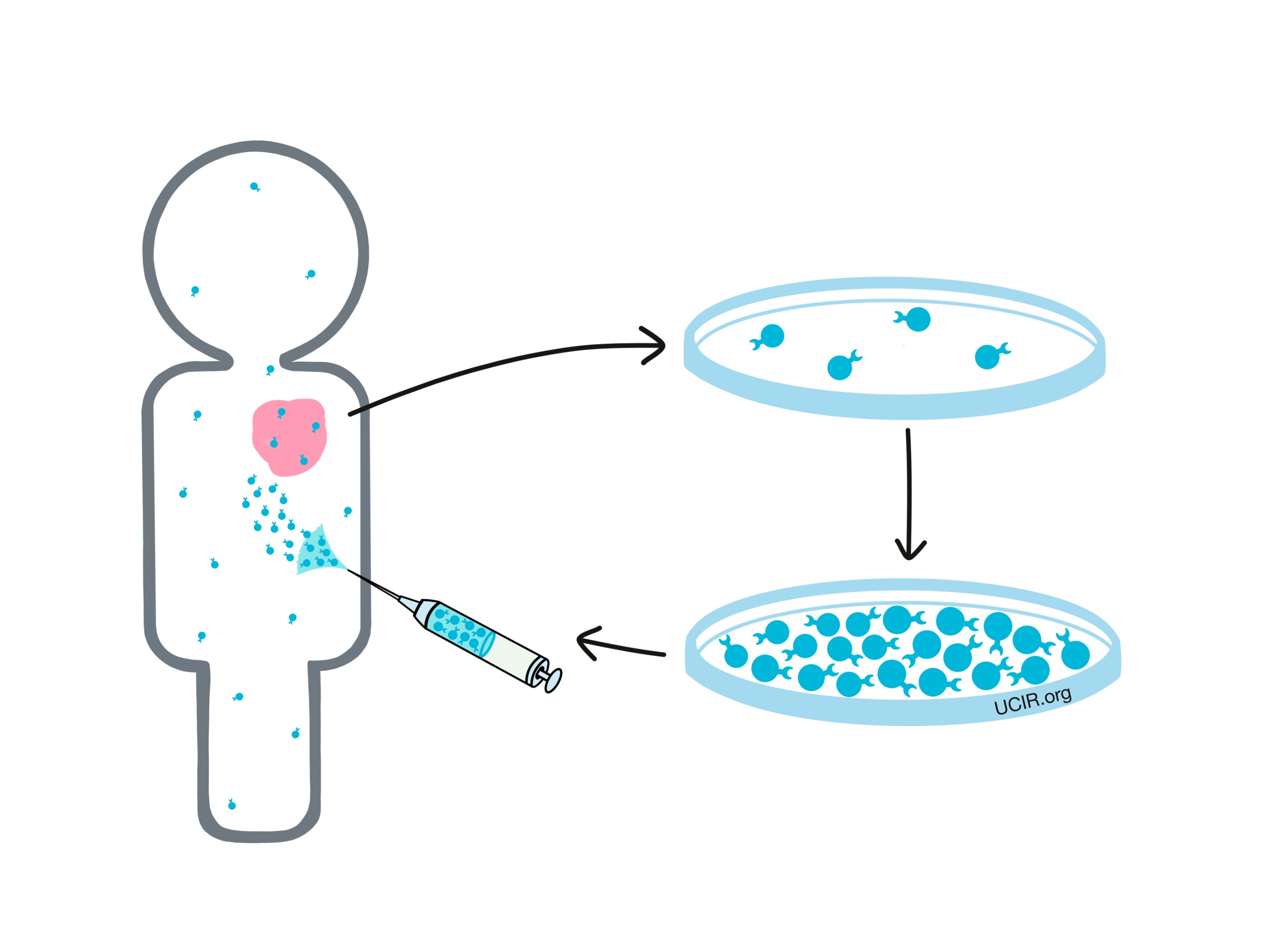
The aim of T cell therapy is to collect some of a patient’s own T cells from their body, grow them in a laboratory to increase their number, and then deliver high numbers of T cells back into the patient. By increasing the number of T cells, the hope is that they will mount a stronger attack on cancer compared to the attack that might occur naturally. Some types of T cell therapy go beyond just increasing the number of T cells, as scientists can actually add in factors or introduce genetic modifications that enhance their cancer-killing functions.
What are the different types of T cell therapy?
Different T cell therapy strategies vary in the source of the T cells, how T cells are treated outside of the body, and the way that T cells recognize the cells they should kill once they are returned to the patient. Naturally, a T cell identifies a target antigen by using receptors on its surface, called T cell receptors. Genetic engineering allows researchers to change which particular antigen will be targeted by a T cell or modify the way how T cells recognize target antigens altogether.
The three main types of T cell therapy that have been used to date are:
- Tumor-Infiltrating Lymphocyte (TIL) therapy: makes use of T cells found within tumors, which may naturally have T cell receptors that recognize targets on cancer cells
- TCR-engineered T cell therapy: makes use of genetic engineering of a patient’s T cells to add alternative T cell receptors that recognize targets on cancer cells
- CAR T cell therapy: makes use of genetic engineering to endow a patient’s T cells with artificial receptors known as “CARs” which can recognize targets on cancer cells that are invisible to T cell receptors.
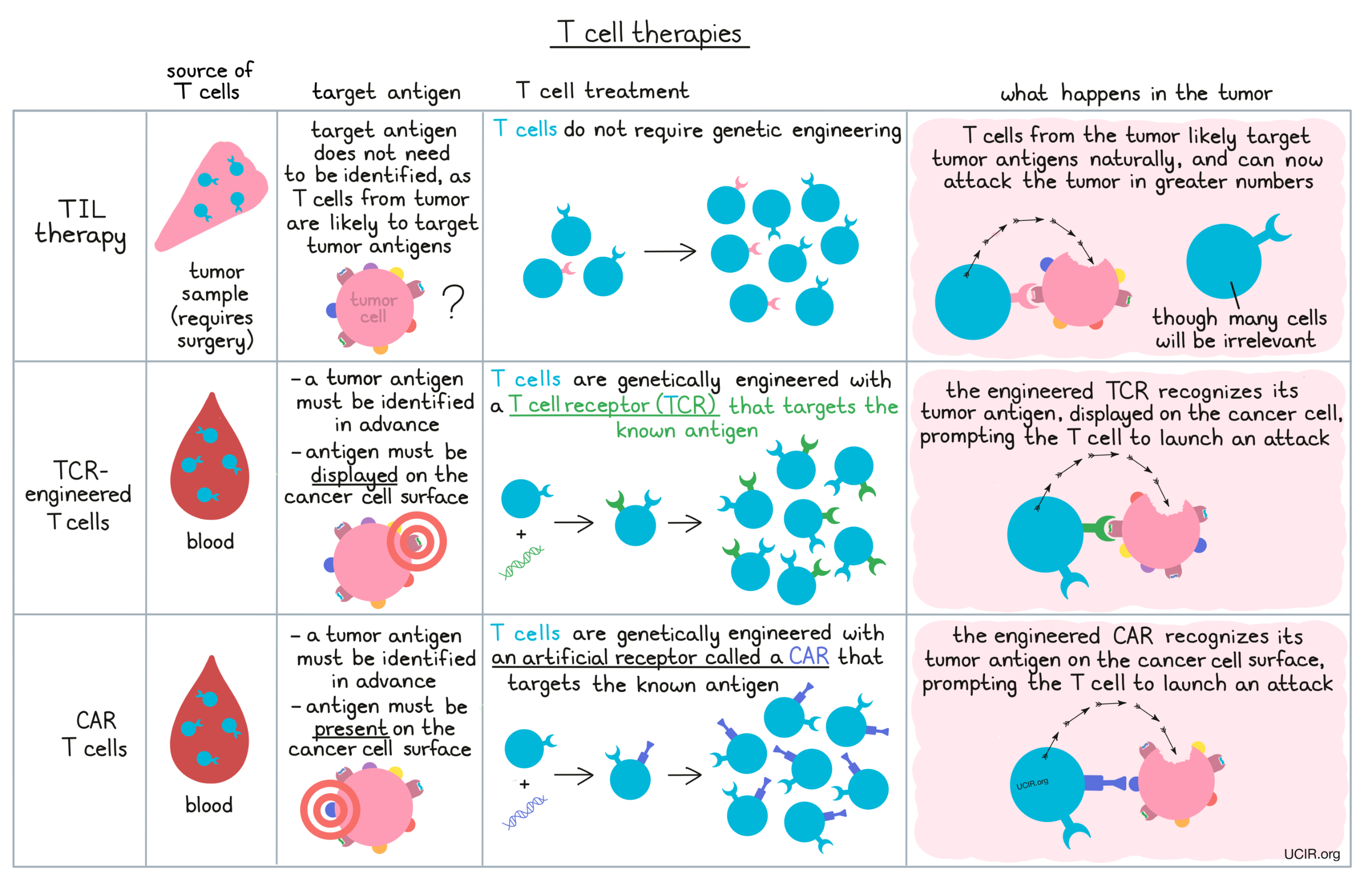
These three types of T cell therapy are discussed in more detail in the following sections.
Tumor-Infiltrating Lymphocyte (TIL) therapy
Tumor-Infiltrating Lymphocyte (TIL)therapy uses immune cells called lymphocytes that had entered the tumor within the patient’s body. A high fraction of these lymphocytes are T cells, the immune cells that are responsible for directly recognizing and killing cells that don’t belong, like cancer cells. The rationale behind TIL therapy is that T cells that are inside a tumor are likely there for a reason, and thus they may be useful in the effort to kill cancer cells.
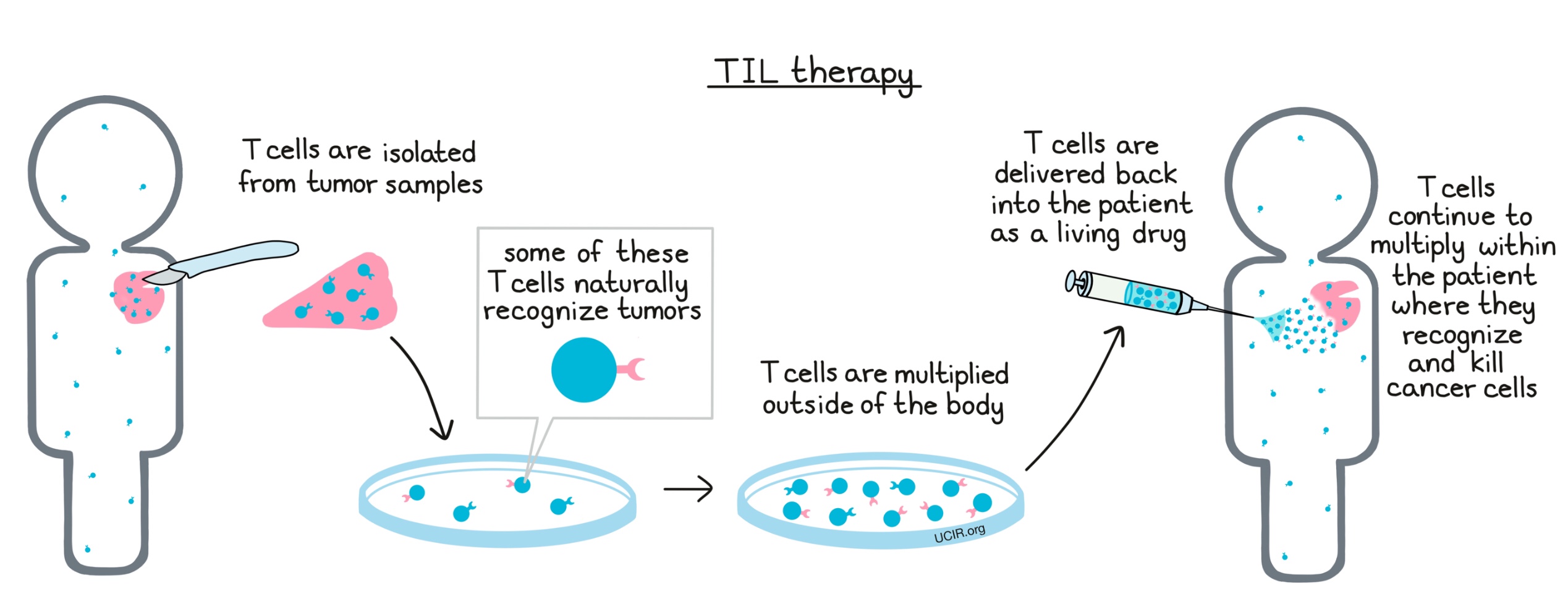
TIL therapy: How it works
To take advantage of the T cells within a patient’s own tumor, a portion or all of the tumor is surgically removed. The T cells from within the tumor sample are then separated out and grown in a laboratory, forming a massive T cell army that can be delivered back into the patient, where it acts as a living drug to kill cancer cells. T cells that are delivered as the drug in TIL therapy are essentially the same as a patient’s own T cells, only more numerous. They use their natural T cell receptors, which are present on their surfaces to recognize molecules on the cancer cells (target antigens) that identify these cells as cancerous. When the T cells encounter cells that display target antigens, they launch a powerful attack to kill them.
TIL therapy: Clinical use
Patients who are candidates for TIL therapy will require a surgery in which all or a portion of the tumor is removed. Preparing the T cell product takes several weeks in a specialized manufacturing facility and is not always successful.
Before T cells are delivered back to the patient, most patients undergo lymphodepletion, meaning that they are treated with chemotherapy (and sometimes low-dose irradiation) to remove most of their natural immune cells. This helps to reduce competition for resources when the laboratory-grown cells are delivered back into the patient, allowing them to better survive and fight the cancer. A few days after lymphodepletion, patients are admitted to the hospital and the TIL therapy is delivered through a vein.
While clinical trials of TIL therapies began in the late 1980s, with several examples of major success stories over the years, the first TIL therapy – called Amtagvi (lifileucel) – was approved by the FDA in 2024 for treatment of patients with advanced melanoma that had gotten worse on previous treatments with targeted therapies or other immunotherapies. In clinical trials, about one third of patients had some sort of response to treatment, with some patients’ tumors disappearing entirely. Research is currently ongoing to improve the efficacy of TIL therapy, find strong combinations with other therapies, and expand the efficacy of TIL therapy to more tumor types and settings.
TIL therapy: Toxicities, Side effects, and Shortcomings
TIL therapy has been effective in a number of patients treated in clinical trials, however, for a patient to be eligible, they must have a tumor from which TILs can be collected.
Lymphodepleting pre-treatment is important for ensuring the survival of the laboratory-grown T cells, but killing off the patient’s natural immune system puts patients at an increased risk of infection. Some other drugs must be delivered in combination with TIL therapy to help the transferred cells survive, and these drugs may come with their own toxicities. TIL therapy requires an extended in-hospital stay during treatment, and the risk of severe toxicities has limited its widespread use.
TIL therapy can fail if:
- the tumor biopsy does not contain a sufficient number of T cells and a TIL therapy product cannot be prepared for the patient
- there are problems growing the T cells in a laboratory
- the patient receives that treatment, but not enough of the T cells in the therapy product are able to recognize cancer cells, or if T cells that do recognize cancer cells are not strong enough to eliminate all of them
- a small portion of the cancer is resistant to therapy, allowing the cancer to live on and possibly grow back
TIL therapy: Next steps
The T cells in a tumor are a mixture of those that recognize the cancer cells and those that do not. Initially, scientists grew all of the T cells from within the tumor sample. Now scientists attempt to identify and expand just the T cells that recognize target antigens that are common across particular cancer types, or target antigens that are unique to an individual patient’s cancer. The hope is that selecting T cells in this way will result in a more potent T cell army and more effective therapy.
Being able to select and multiply only the T cells that recognize the cancer cells also opens up the possibility that in the future, researchers could identify these rare T cells in the blood, sparing patients from invasive surgery.
TCR-engineered T cells
T cells are already present throughout a patient’s body. While some T cells may naturally have the ability to recognize and kill cancer cells, most are only programmed to recognize other targets like bacteria or viruses. T cell receptor (TCR) engineering allows researchers to control what a T cells targets, meaning that they can train the T cells to recognize and attack cancer cells.
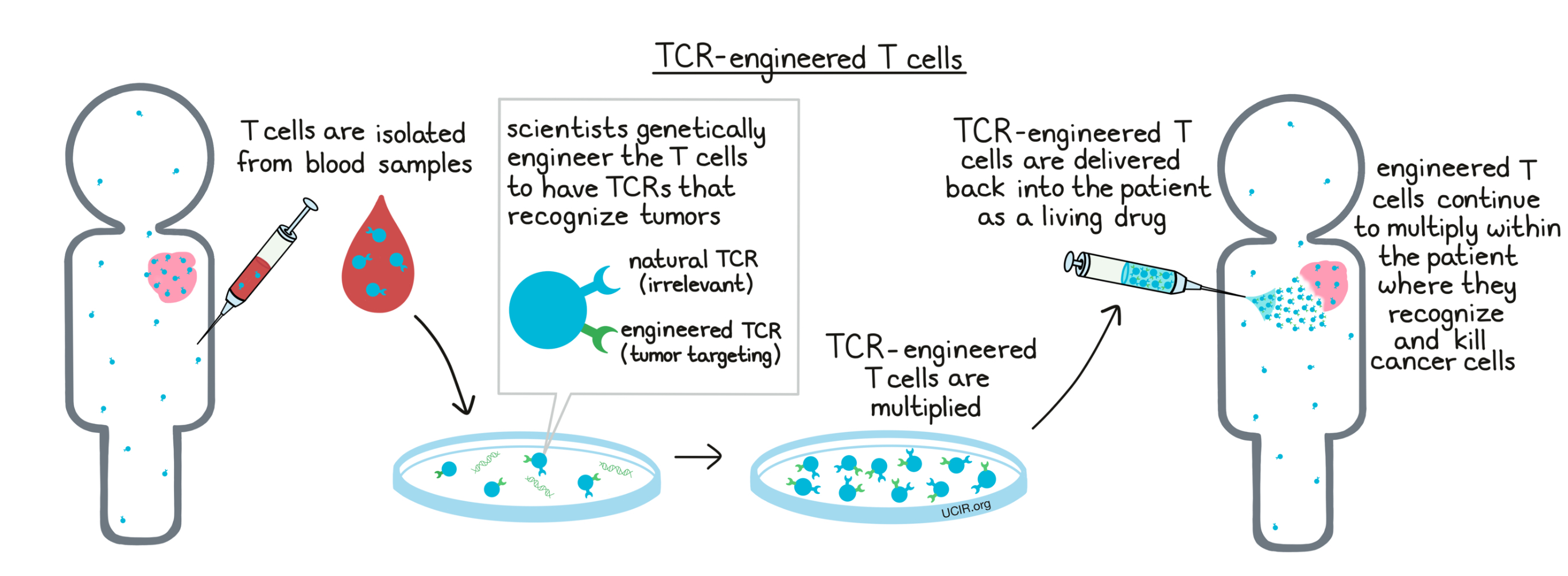
TCR-engineered T cells: How they work
T cells have the ability to recognize and kill unhealthy cells by identifying molecules called antigens that aren’t normally present in healthy cells. A T cell identifies an antigen using receptors on its surface called T cell receptors (TCRs). T cells patrol the body at all times, looking for any threats that they recognize, and when they find their known target, the T cells launch an attack.
In the case of cancer, scientists can identify a marker on the cancer cells, and can design a T cell receptor that recognizes it. Making TCR-engineered T cells to use as a drug involves taking a blood sample from a patient, collecting T cells from within the blood, and adding in genes that code for the T cell receptor that will directly match up with the cancer cells. This essentially gives the patient’s own cells the ability, that they did not have before, to recognize cancer cells. These TCR-engineered T cells can be grown and multiplied in a laboratory to form a massive T cell army that can then be delivered back into the patient, where it can act as a living drug that recognizes and kills off cancer cells within the patient.
TCR-engineered T cells: Clinical use
TCR-engineered T cell therapy is usually utilized in patients with very late-stage cancers. To be eligible for treatment with TCR-engineered T cells, the presence of the target antigen in the patient’s cancer cells must be confirmed using laboratory tests.
Patients who are candidates for TCR-engineered T cell therapy undergo a procedure known as leukapheresis in which their blood system is connected for about 3 hours to a machine that carefully collects a large number of immune cells, including T cells. The cells are sent to a special manufacturing facility where the T cells are separated out. Scientists insert the gene into the T cells that codes for a T cell receptor capable of recognizing the target antigen that was previously confirmed to be present in the patient’s cancer cells. These TCR-engineered T cells are increased in number and delivered back to the patient. The manufacturing process can take several weeks.
Before T cells are delivered back to the patient, most patients undergo lymphodepletion, meaning that they are treated with chemotherapy (and sometimes low-dose irradiation) to remove most of their natural immune cells. This helps to reduce competition for resources when the laboratory-made cells are delivered back into the patient, allowing the laboratory-made cells to better survive and fight the cancer. A few days after lymphodepletion, patients are admitted to the hospital and the TCR-engineered T cell therapy is delivered through a vein.
In 2024, the first TCR-engineered T cell therapy was approved under the drug name Tecelra (afamitresgene autoleucel or afami-cel) for certain patients with synovial sarcoma. Tecelra specifically recognizes the MAGE-A4 antigen that is present in the patient’s cancer cells. Clinical trials are currently ongoing for numerous other TCR-engineered T cell therapies targeting various antigens on a wide range of tumor types.
TCR-engineered T cells: Toxicities, Side effects, and Shortcomings
Identifying target antigens that are present exclusively in a patient’s cancer cells is one of the major challenges in TCR-engineered T cell therapy. The most significant toxicity observed so far with TCR-engineered T cell therapy occurs when TCR-engineered T cells attack normal cells that display the target antigen or a molecule that closely resembles the target antigen. Tests to predict such off-target attacks have not yet proven reliable.
Lymphodepleting pre-treatment is important for ensuring the survival of the laboratory-grown T cells, but killing off the patient’s natural immune system puts patients at an increased risk of infection.
Some drugs that help the laboratory-made cells to survive in the patient may also cause toxicity and may require hospitalization during use.
TCR-engineered T cell therapy can fail if:
- tumor antigens are present in or on the tumor, but are invisible to T cells because they are not displayed in a way that the T cells can “see”
- the cancerous cells are not all the same. Even if most of the cancer cells contain antigens that can be recognized by TCR-engineered T cells, some cancer cells may not contain the antigen, allowing them to hide, escape destruction, and live on in the patient.
TCR-engineered T cells: Next steps
Recent advances in the ability to identify tumor antigens called neoantigens, which are very unique to the patients’ cancer cells, may help to improve TCR-engineered T cell therapy and prevent damage to healthy cells and tissues.
Because TCR-engineered T cells are designed to stay alive in a patient’s body, toxicity that develops (like attacks on normal, healthy cells) is difficult to stop. Researchers are currently developing engineered T cells that have an “off” switch in case toxicity develops.
In current T cell TCR engineering strategies, the original T cell receptor is left intact on the T cell’s surface. The original T cell receptor may compete with the new T cell receptor for space on the T cell surface. The two receptors may also fuse together, which can make the new receptor less effective and can lead to toxicity. A number of ongoing studies are focused on eliminating the natural T cell receptor to address these problems.
CAR T cells
CAR T cells are a type of T cell therapy in which T cells are modified with an artificial receptor known as chimeric antigen receptor, or CAR, to recognize cancer cells. While natural T cells have T cell receptors (TCRs), that allow them to “see” a wide range of molecules on cells that might identify those cells as cancerous, the T cells can’t see all of the molecules present on a cancer cell’s surface. A CAR is an artificially-produced receptor that can act a lot like a T cell receptor, except that the way it “sees” antigens is different. In this way, a T cell can be engineered to “see” molecules on a cancer cell that are invisible to natural T cells. The ability to generate CAR T cells therefore broadens the number of antigens that can be targeted, the number of cancer cells that can be killed, and the number of patients that can benefit from T cell therapy.
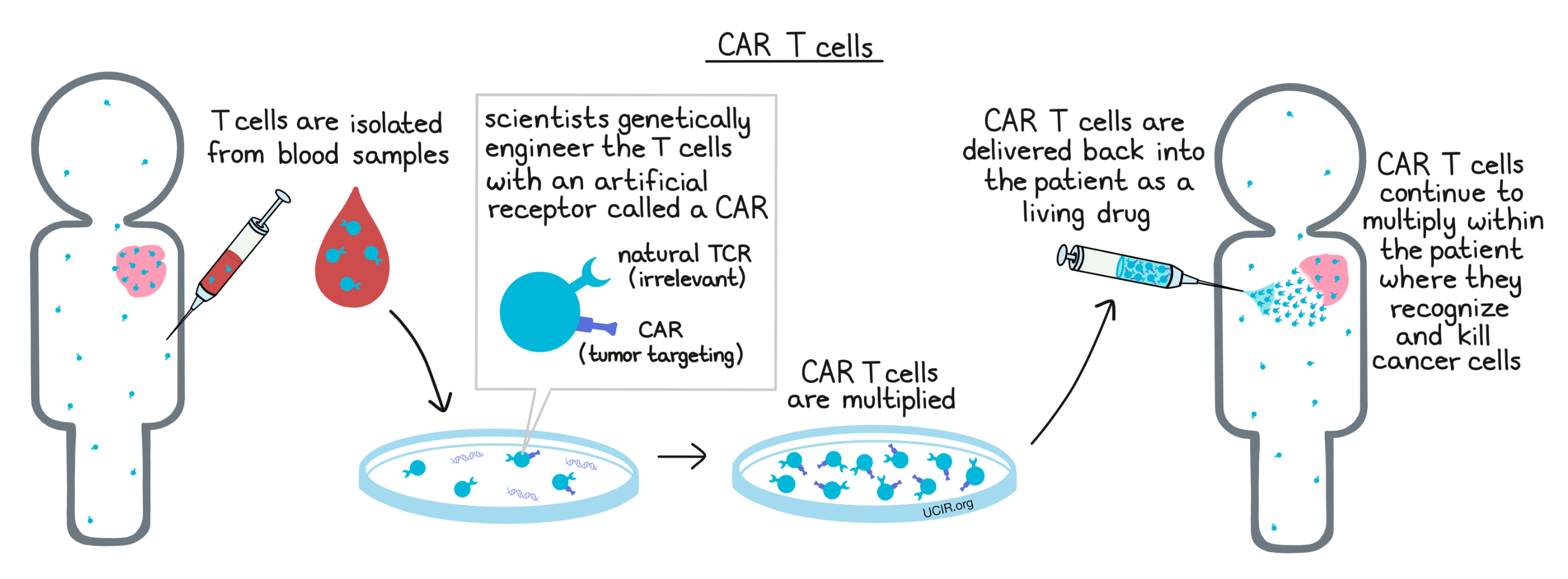
CAR T cells: How they work
The first, and most critical, step in generating a CAR is to identify a target antigen that is present on the surface of cancer cells, but not on any healthy cells that are essential to the patient. Then scientists can design a CAR, which combines the abilities of an antibody and a T cell receptor. The antibody part of the CAR allows this receptor to “see” like an antibody, meaning that it can “see” antigens directly on the surfaces of cells. The T cell receptor part of this frankenstein receptor triggers the T cell to attack when the antibody part has found a match. CAR T cells are generated by inserting the gene that codes for the CAR into T cells that have been isolated from a patient’s own blood. When CAR T cells are delivered back into the patient, they target the specific cancer cell antigen they are programmed to recognize and attack.
CAR T cells: Clinical use
CAR T cell therapy is usually utilized in patients with very late-stage cancer.
To be eligible for treatment with CAR T cells, a target antigen must be identified on a patient’s cancer cells using laboratory tests.
Patients who are candidates for CAR T cell therapy undergo a procedure known as leukapheresis in which their blood system is connected for about 3 hours to a machine that carefully collects a large number of immune cells from the blood. These cells are sent to a special manufacturing facility where the T cells are isolated and the gene for the CAR is inserted. The gene then acts as a “construction plan” for the cells to build the CARs and to display them on their surfaces. The cells are then increased in number in a laboratory before being delivered back to the hospital where they can be administered to the patient. The manufacturing process can take several weeks.
Before the CAR T cells are delivered back to the patient, most patients undergo lymphodepletion, meaning that they are treated with chemotherapy to remove most of their natural immune cells. This helps to reduce competition for resources when the laboratory-made cells are delivered back into the patient, allowing the laboratory-made cells to better survive and fight the cancer.
A few days after lymphodepletion, patients are admitted to the hospital and the CAR T cell therapy is delivered through a vein. Side effects often begin within days of treatment, and depending on their condition, patients may need to remain in the hospital for a few weeks to monitor their side effects. Additional drugs may be given to manage side effects and complications. Patients who are released from the hospital should plan to remain close to their treatment facility for at least 30 days to continue to monitor side effects and progress. Some patients may need to be readmitted to the hospital during that period.
Several CAR T cell drugs are currently approved for clinical use against a range of blood cancers, including large B-cell lymphomas, chronic lymphocytic lymphomas (CLL), follicular lymphomas (FL), mantle cell lymphomas (MCL), B-cell acute lymphoblastic leukemia (ALL), and multiple myeloma (MM). Currently approved drugs include Tecartus, Yescarta, Carvykti, Abecma, Breyanzi, and Kymriah.
CAR T cells: Toxicities, Side effects, and Shortcomings
CAR T cell therapy requires a known cancer antigen and identifying the right one can be a challenge. For many cancer types, it is difficult to find a good target that is exclusive to the cancer cells, limiting the number of patients who are eligible for this therapy. Further, CAR T cells can only recognize antigens that are present on the surface of a cancer cell – they cannot “see” antigens that might be hidden inside of a cell, limiting the list of potential targets.
Lymphodepleting pre-treatment is important for ensuring the survival of T cells, but killing off the patient’s natural immune system puts patients at an increased risk of infection.
When large numbers of CAR T cells launch an attack on tumors, the body might react with strong inflammatory responses, leading to a potentially life-threatening condition known as cytokine release syndrome. This condition requires immediate treatment with powerful anti-inflammatory drugs. Patients who experience a life-threatening case of cytokine release syndrome may suffer from long-term effects, including neurological problems.
Currently, CAR T cells are most commonly used to treat B cell blood cancers. So far, the best target for B cell cancers is an antigen that is present on all B cells – cancerous or not – known as CD19. While CAR T cells kill off all of the cancerous B cells in a patient’s body, they also kill off the healthy B cells (temporarily or sometimes permanently). Patients can live without B cells, but this can leave them vulnerable to infections. Many patients require antibody replacement infusions for life to replace what would normally be produced by healthy B cells.
Thus far, CAR T cell therapies have been highly effective in treating certain types of blood cancer, but have shown little effect against solid tumors.
Although CAR T cell therapy in B cell cancers is very effective in eliminating visible disease, the cancer can relapse, most often due to growth of cancer cells that do not contain the targeted antigen and are therefore missed by the CAR T cells. Fully killing of hidden cancer cells is a major unmet need for CAR T cell therapy and a focus of active research.
CAR T cell therapy is very expensive compared to other cancer therapies.
CAR T cells: Next steps
The success of CAR T cells in certain B cell cancers has prompted a massive amount of CAR T cell research. Researchers are now focusing on developing CAR T cell therapies for more cancer types (beyond just blood cancers), and on making CAR T cells that are more potent and less toxic. They are also working on making sure that CAR T cells don’t leave any hidden cancer cells behind; this may be done by using mixtures of CAR T cells that target different antigens, or by designing CAR T cells that can target multiple antigens at once. Many researchers also hope to utilize CAR T cells in combinations with other effective immunotherapies and are searching for safe combination treatments that work together to eliminate cancer. Hundreds of clinical trials involving CAR T cells are currently underway.
Because CAR T cell therapy is a “living drug”, designed to persist in the body, it cannot be easily stopped if toxicity due to attacks on normal, healthy tissue develops. Researchers are developing multiple types of “off” switches which will allow them to specifically kill or “turn off” the CAR T cells and reduce toxicity if it develops.
In current CAR T cell strategies, the original T cell receptor is left intact on the T cell’s surface. The original T cell receptor may compete with the CAR for space on the T cell’s surface, making the new receptor less effective. A number of ongoing studies are focused on eliminating the natural T cell receptor.
While T cells are often considered the main tumor-killing immune cell, natural killer (NK) cells can also launch attacks against cancer cells, and in some cases, there may be advantages to using NK cells instead of T cells. Modifying NK cells with a CAR may allow researchers to utilize this powerful alternative immune cell to target and kill cancer. Several clinical trials using CAR NK cells are currently underway.
Further information
UCIR news story:
Videos:
- T-Cell Transfer Therapy, National Cancer Institute
- T-cell therapy for cancer treatment: How it works, MD Anderson Cancer Center
- CAR T-Cell Therapy: How Does It Work?, Dana-Farber Cancer Institute
- How CAR T-Cell Therapy Works: An Animation for Kids, The Children’s Hospital of Philadelphia
Scientific references:
- Adoptive cell transfer as personalized immunotherapy for human cancer (Review article), Rosenberg SA, Restifo NP. Science (2015).
- Adoptive transfer of tumor-infiltrating lymphocytes in melanoma: a viable treatment option (Review article), Rohaan MW, van den Berg JH, et al. Journal for Immunotherapy of cancer (2018).
- Emerging Cellular Therapies for Cancer (Review article), Guedan S, Ruella M, June CH. Annual Review of Immunology (2018).