How is this drug name pronounced?
Blinatumomab: Blin-a-TOOM-oh-mab
Blincyto: Blin-SITE-oh
What cancer(s) does this drug treat?
B-precursor Acute Lymphoblastic Leukemia (ALL)
Blincyto is approved for:
- Adults and children with B-precursor Acute Lymphoblastic Leukemia (B-ALL) for whom chemotherapy was mostly successful, but a small number of cancer cells still remain (a condition known as Minimal Residual Disease [MRD]). These cells may put the patient at an increased risk of relapse, and Blincyto can be prescribed to eliminate all remaining traces of the cancer. The cancer cells must express the CD19 molecule on their surface.
- Adults and children with B-precursor Acute Lymphoblastic Leukemia (B-ALL) who had been treated with chemotherapy, targeted therapy or allogeneic stem cell transplantation, but the cancer either did not respond to treatment (refractory) or has since returned (relapsed). The cancer cells must express the CD19 molecule on their surface.
- Adults and children with B-precursor Acute Lymphoblastic Leukemia (B-ALL) that is negative for the Philadelphia chromosome. In such cases, Blincyto is used in the consolidation phase of multiphase chemotherapy. The cancer cells must express the CD19 molecule on their surface.
Limitations of use
Neonates: For the treatment of neonates, preservative-free formulations of Blincyto should be used.
Pregnancy/breastfeeding: Blincyto is not recommended for use in women who are pregnant or breastfeeding. Appropriate contraceptive measures should be used by patients of child-bearing potential while receiving Blincyto, and for two days after the last dose. Due to the potential for harm in a breastfed child, patients should not breastfeed during treatment with Blincyto or for two days after the last dose of Blincyto.
Effects on the ability to drive and use machines: Patients are advised to refrain from driving, engaging in hazardous occupations or activities, and operating heavy machinery during therapy with Blincyto.
What type of immunotherapy is this?
- Bispecific antibody therapy
How does this drug work?
Targets:
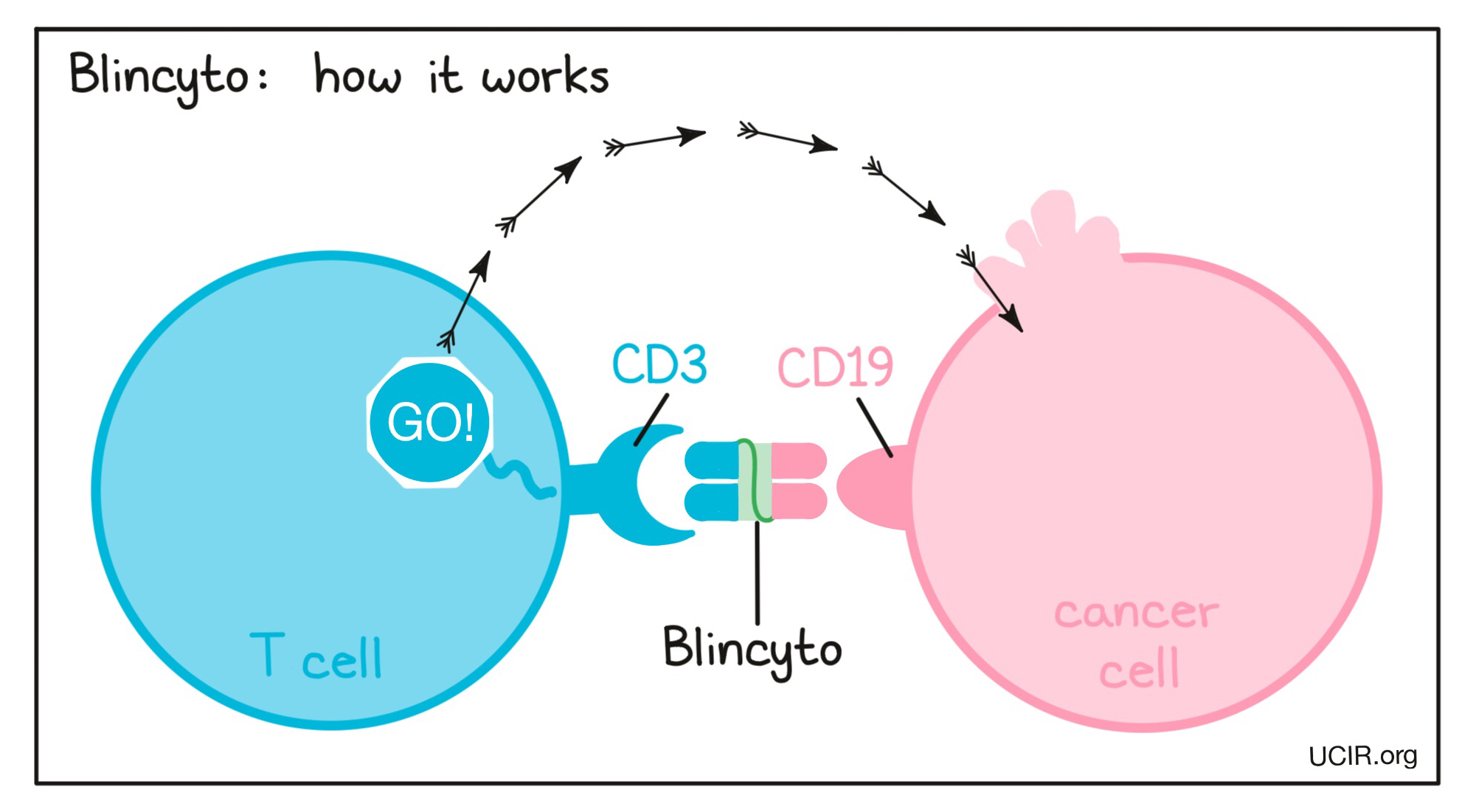
Blincyto is a “bispecific antibody”. Antibodies are molecules which can bind to a particular target molecule. Bispecific antibodies are artificially made in the laboratory and can bind to two different targets at the same time. Blincyto binds to:
- a molecule called CD19 on the surface of a leukemic B cell. CD19 is found on the surface of all human B cells, where it is almost always present due to its importance to their normal function, however it is also present on the surface of B-precursor ALL cells.
- a molecule called CD3 on the surface of a T cell – the primary immune cell involved in killing cancer cells. CD3 is part of the T cell receptor, which is critical to the function of the T cell and is involved in stimulating the T cell to become active.
Because Blincyto can bind to one molecule on leukemic B cells and one on T cells at the same time, it acts as a bridge and keeps the T cell in close contact with the leukemic B cell. By binding CD3 on the T cell, Blincyto also stimulates the T cell to become activated and kill nearby cells. Bispecific antibodies that direct T cells to kill cancer cells by binding to both cells at the same time are known as bispecific T cell engagers (BiTEs).
How is the drug given to the patient?
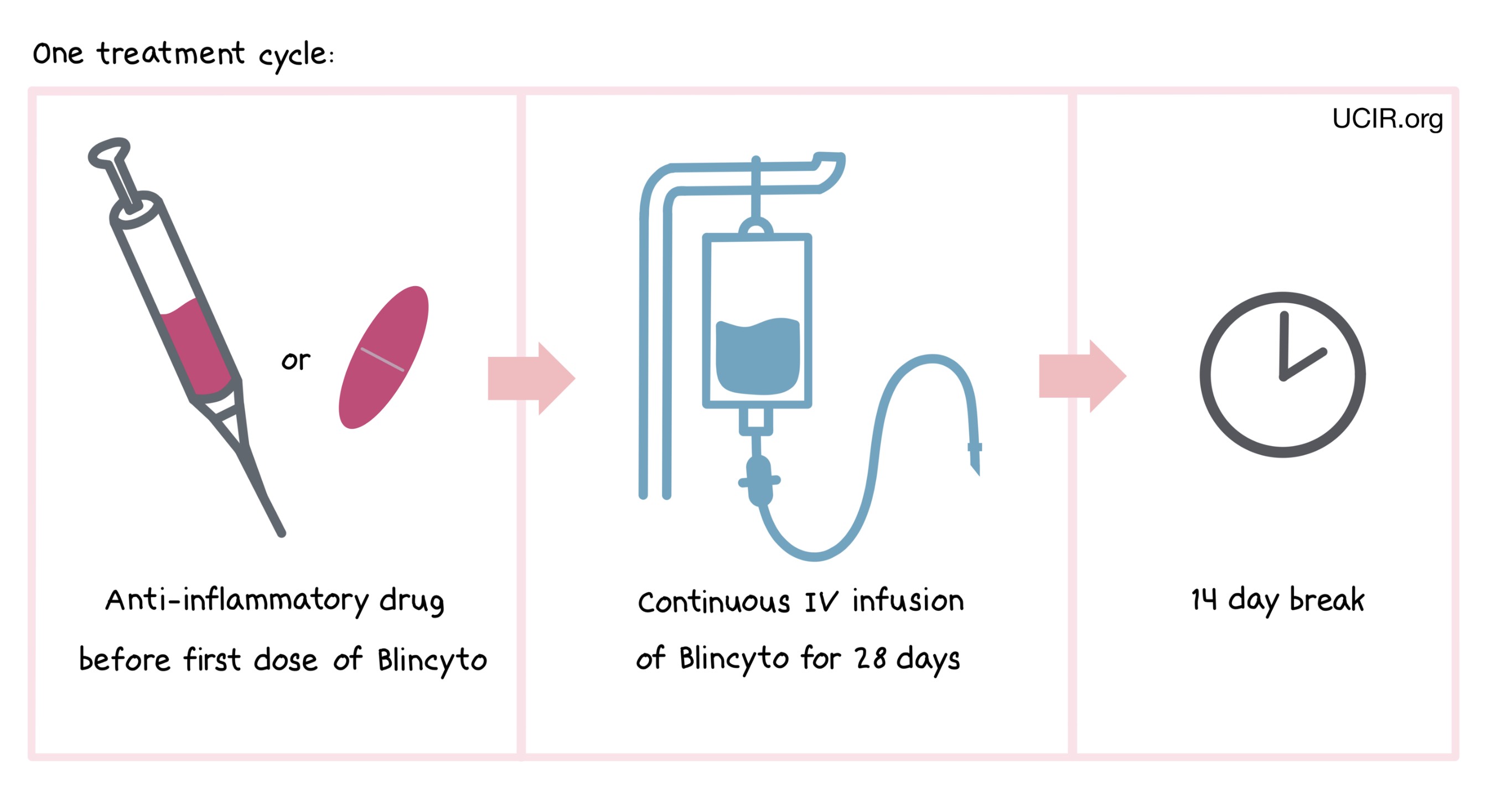
Blincyto is administered via a tube into a vein (intravenous infusion, or I.V.) continuously for 28 days, followed by a break of 14 days. The combined period of the 28-day treatment and the 14-day break is one “cycle”. Blincyto is administered across multiple treatment cycles.
One hour prior to the first dose of Blincyto in each cycle, adult patients are pre-medicated with an anti-inflammatory drug (prednisone or dexamethasone). Pediatric patients are pre-medicated with the anti-inflammatory drug dexamethasone prior to the first dose of Blincyto in the first cycle, and when restarting an infusion after any interruptions of 4 or more hours in the first cycle.
Hospitalization is recommended for the first 9 days of the first cycle for patients with relapsed/refractory B-ALL (who had been treated with chemotherapy, but it either did not work or stopped working) and the first 3 days of the first treatment cycle for patients with minimal residual disease B-ALL or Philadelphia chromosome-negative B-ALL. For subsequent cycles, fewer hospitalization days are required. Hospitalization or supervision by a healthcare professional is also recommended for all subsequent cycle starts, and when restarting an infusion after any interruptions.
The first treatment cycle (for patients with minimal residual disease B-ALL) or the first two cycles (for patients with relapsed/refractory B-ALL) are intended to drastically reduce or eliminate leukemic cells and decrease cancer-associated symptoms. These are called “induction cycles”. After the induction cycle(s), up to three more cycles are administered in order to further reduce the leukemic cells and prevent the disease from returning after discontinuing Blincyto. These are called “consolidation cycles”. Patients with relapsed/refractory B-ALL can then receive up to 4 additional cycles of Blincyto with a break of 56 days (“continued therapy”). Patients with Philadelphia chromosome-negative B-ALL receive one consolidation cycle of Blincyto.
What are the observed clinical results?
For: MRD-positive B-ALL
Relapsed or refractory B-ALL
Philadelphia chromosome-negative B-ALL in the consolidation phase
It is important to keep in mind that each patient’s outcome is individual and may be different from the results found in the clinical studies. In addition, immunotherapy can sometimes take several months to yield an observable treatment response.
MRD-positive B-precursor Acute Lymphoblastic Leukemia (B-ALL)
In a clinical trial of 86 patients with minimal residual disease (MRD) B-ALL, leukemia cells could no longer be detected in 81% of the patients after one treatment cycle with Blincyto. The median time for the leukemia to come back was 22 months. For patients in first remission (who had achieved minimal residual disease status for the first time) treatment with Blincyto prevented the leukemia from returning for a median of 35 months.
Relapsed or refractory B-precursor Acute Lymphoblastic Leukemia (B-ALL)
In a clinical trial, 405 adult patients with relapsed or refractory Philadelphia chromosome-negative B-ALL (who had been treated with chemotherapy or allogeneic stem cell transplantation, but it either did not work or stopped working), were treated with Blincyto or a physician’s choice of any of 4 standard-of-care chemotherapies. Patients treated with Blincyto lived for a median of 8 months, while patients treated with standard-of-care chemotherapy lived for a median of 4 months. 42% of patients treated with Blincyto had their leukemia completely disappear (complete remission), compared to 20% of patients achieving complete remission with standard-of-care chemotherapy.
In another clinical trial, 185 adult patients with relapsed or refractory B-ALL were treated with Blincyto. 42% of patients had their leukemia completely disappear following treatment. The median time for the leukemia to come back was 6 months.
In another clinical trial, 45 adult patients with Philadelphia chromosome-positive B-ALL who had been previously treated with imatinib (Gleevec) and other targeted therapies, but for whom treatment either did not work, stopped working, or was stopped because it caused severe side effects for the patient, were treated with Blincyto. 36% of patients had their leukemia completely disappear (complete remission). The remission lasted for a median of 7 months.
In another clinical trial, 70 pediatric patients (under 18 years of age) with B-ALL that had returned or had not responded to previous treatments, were treated with Blincyto. 33% of patients had their leukemia completely disappear (complete remission). The remission lasted for a median of 6 months.
Philadelphia chromosome-negative B-cell Precursor ALL in the consolidation phase
In a clinical trial, 224 adult patients with newly diagnosed Philadelphia chromosome-negative B-cell precursor ALL who were in complete remission or complete remission with incomplete blood count recovery following chemotherapy were treated with consolidation therapy consisting of either Blincyto plus chemotherapy or chemotherapy alone to keep the cancer from coming back. Among patients who received Blincyto plus chemotherapy, 85% were alive at the 3-year mark and 82% were alive at the 5-year mark, while among patients who received chemotherapy alone, 69% were alive at the 3-year mark and 63% were alive at the 5-year mark.
In another clinical trial, 111 pediatric patients (1 month to 18 years old) with Philadelphia chromosome-negative B cell-precursor ALL that had come back after one prior treatment, but were down to a low level of disease after two treatment cycles of consolidation chemotherapy were treated with Blincyto or with an intensive combination chemotherapy as their third consolidation therapy cycle prior to allogeneic stem cell transplantation. Among patients who received Blincyto, 78% were alive at the 5-year mark and 61% had not experienced return of their disease (relapse), while among patients who received chemotherapy, 41% were alive at the 5-year mark and 28% had not experienced relapse.
What are the potential side effects?
The most common side effects of Blincyto include fever, infusion-related reactions, infections, headache, nausea, diarrhea, muscle, joint and bone pain, low red and white blood cell counts, and low platelet counts.
Blincyto targets the CD19 molecule, which, while present on leukemic B cells, is also present on normal B cells. As a result, Blincyto can kill normal B cells, increasing the risk of serious infections. Other side effects, such as pancreatitis, cytokine release syndrome (CRS), neurological toxicities, and tumor lysis syndrome can become serious or life-threatening. Patients and caregivers receive careful instructions to monitor for signs and symptoms related to these side effects, and each of these conditions are managed by the health care provider.
Cytokine release syndrome (CRS)
CRS is caused by a widespread release of molecules called cytokines, which can cause inflammation and can affect the function of various organs. Cytokines may be released by the T cells to which Blincyto binds, or by other immune cells in the patient’s body. Signs and symptoms of CRS include fever, headache, nausea, low blood pressure, and weakness. CRS typically occurs 2 days after the start of Blincyto infusion. A healthcare provider should be immediately notified if symptoms occur.
Neurological toxicities Some of the cytokines released during CRS can result in disruption of the blood-brain barrier, leading to the development of neurological toxicities (including immune effector cell-associated neurotoxicity syndrome [ICANS]). Symptoms of neurological toxicities include headache, tremors, seizures, loss of consciousness, confusion, difficulty with speech, and loss of balance. Neurological toxicities typically occur within the first two weeks of treatment with Blincyto.
Tumor lysis syndrome (TLS) TLS is toxicity caused by the rapid breakdown of cancer cells. It can be severe or life-threatening. Symptoms of TLS include nausea, vomiting, confusion, shortness of breath, irregular heartbeat, dark or cloudy urine, reduced urine production, unusual tiredness, and muscle cramps.
For a more complete list of possible side effects, see the full prescribing information.
Additional information
Manufacturer
Amgen
Approval
FDA and EMA
Links to drug websites
Last updated: September 17, 2024


