How is this drug name pronounced?
Generic name: seh-MIP-lih-mab
Brand name: lib-TY-oh
What cancer(s) does this drug treat?
Libtayo is approved for:
Cutaneous squamous cell carcinoma
Basal cell carcinoma
Non-small cell lung cancer
Cutaneous squamous cell carcinoma
Libtayo is approved for:
- Patients with squamous cell skin cancer that has grown (locally advanced) or spread (metastatic), and who cannot receive surgery or radiation.
Basal cell carcinoma
Libtayo is approved for:
- Patients with basal cell carcinoma that has grown into nearby tissue (locally advanced) OR has spread to other parts of the body (metastatic), and who have been previously treated with a hedgehog pathway inhibitor (such as sonidegib [Odomzo] or vismodegib [Erivedge]) or who cannot be treated with a hedgehog pathway inhibitor.
Non-small cell lung cancer
Libtayo is approved for:
- Patients with non-small cell lung cancer that:
-
- tests positive for high levels of the PD-L1 molecule, AND
- does not have an abnormal EGFR, ALK, or ROS1 gene, AND
- either has not spread outside the chest, but cannot be treated with surgery or chemotherapy with radiation, OR has spread outside the chest.
In such cases, Libtayo may be used alone as a first treatment of the advanced disease.
In such cases, Libtayo may be used in combination with platinum-based chemotherapyas a first treatment for the advanced disease.
Limitations of use:
Age: The safety and efficacy of Libtayo in patients under 18 years of age have not been established.
Pregnancy/Breastfeeding: Libtayo can cause harm to a fetus, and is not recommended for use during pregnancy. The risks associated with Libtayo during breastfeeding are not known and cannot be ruled out; due to the potential for serious adverse reactions to the breastfed child, women are advised not to breastfeed during treatment and for at least 4 months after the last dose of Libtayo.
Complications of stem cell transplant: Serious and life-threatening complications that can lead to death can occur in patients who have received a stem cell transplant from a stem cell donor before or after being treated with Libtayo.
What type of immunotherapy is this?
How does this drug work?
Libtayo is an antibody that attaches to a molecule called PD-1, which is present on the surface of T cells – the primary immune cells involved in killing cancer cells. In healthy tissues, PD-1 acts as a brake that keeps T cells from creating an immune reaction that gets out of control. However, cancers can hijack this safety mechanism and prevent T cells from doing their job – killing the cancer cells. This can happen when cancer cells or other cells within the tumor mass display on their surface molecules called PD-L1 or PD-L2. When PD-L1 or PD-L2 interact with PD-1 on T cells, the T cells become inactive and do not attack the cancer cells. Libtayo binds to the PD-1 molecules on T cells in such a way that prevents the interaction between PD-1 and PD-L1/PD-L2, and allows the T cells to be active and attack the cancer cells.
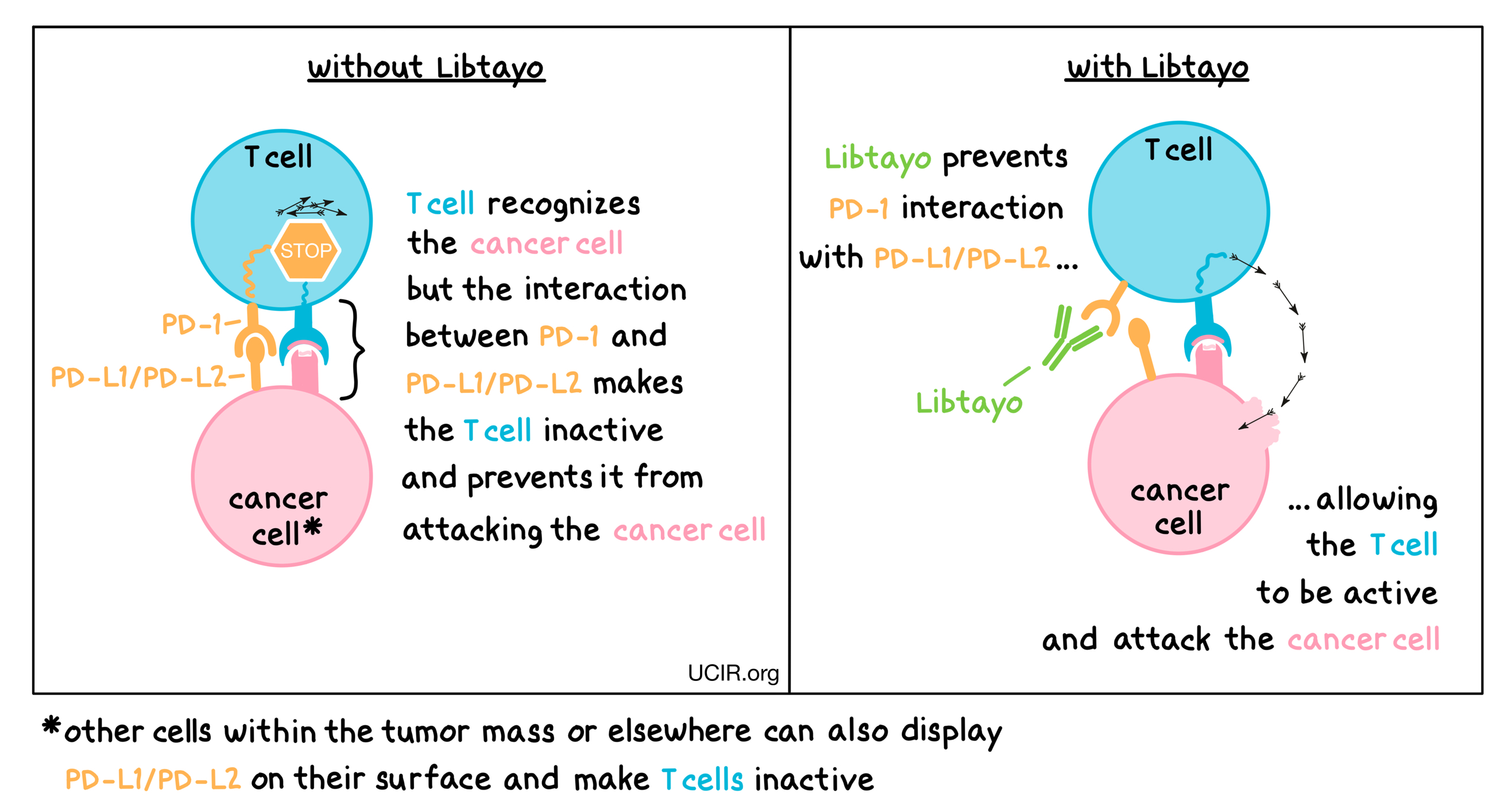
How is this drug given to the patient?
Libtayo is administered via a tube into a vein (intravenous infusion, or i.v.) over 30 minutes every three weeks and does not require a hospital stay.
What are the observed clinical results?
For:
Cutaneous squamous cell carcinoma
Basal cell carcinoma
Non-small cell lung cancer
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Cutaneous squamous cell carcinoma
In a clinical trial, 193 patients with squamous cell skin cancer who could not receive surgery or radiation were treated with Libtayo every two or three weeks.
Among 78 patients with squamous cell skin cancer that had grown at the initial tumor site who were treated with Libtayo every 2 weeks, at median follow-up of 16 months:
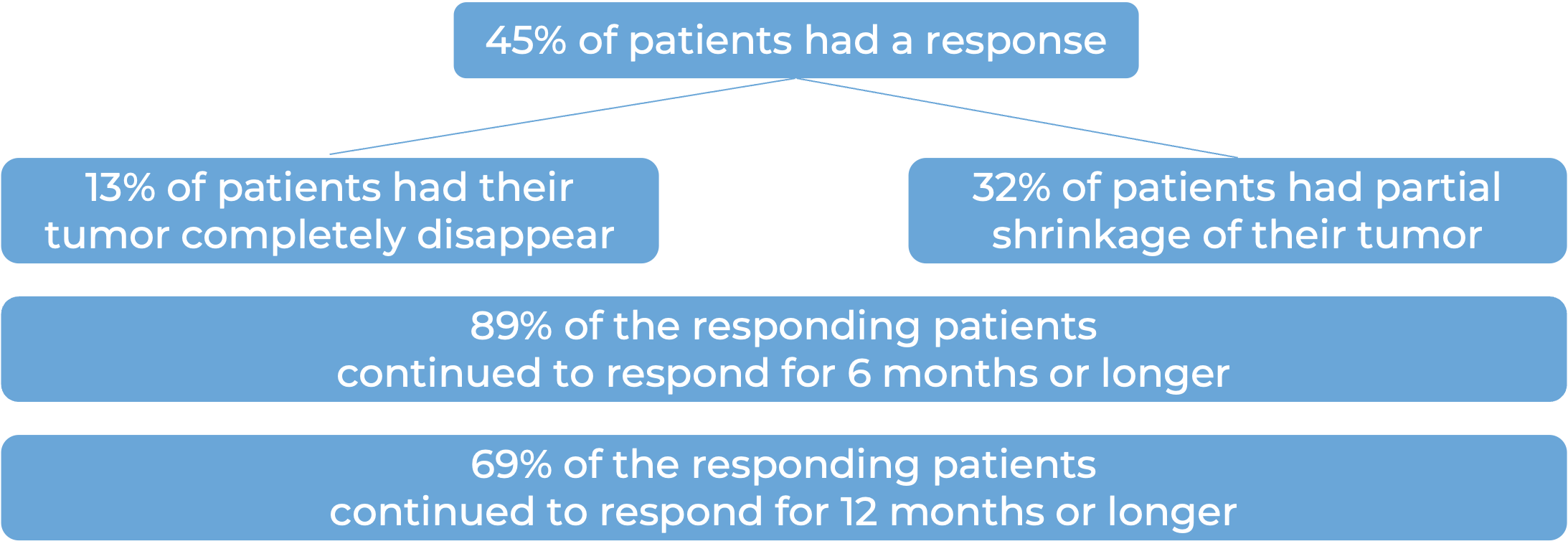
Among 59 patients with squamous cell skin cancer that had spread to other parts of the body who were treated with Libtayo every 2 weeks, at median follow-up of 19 months:
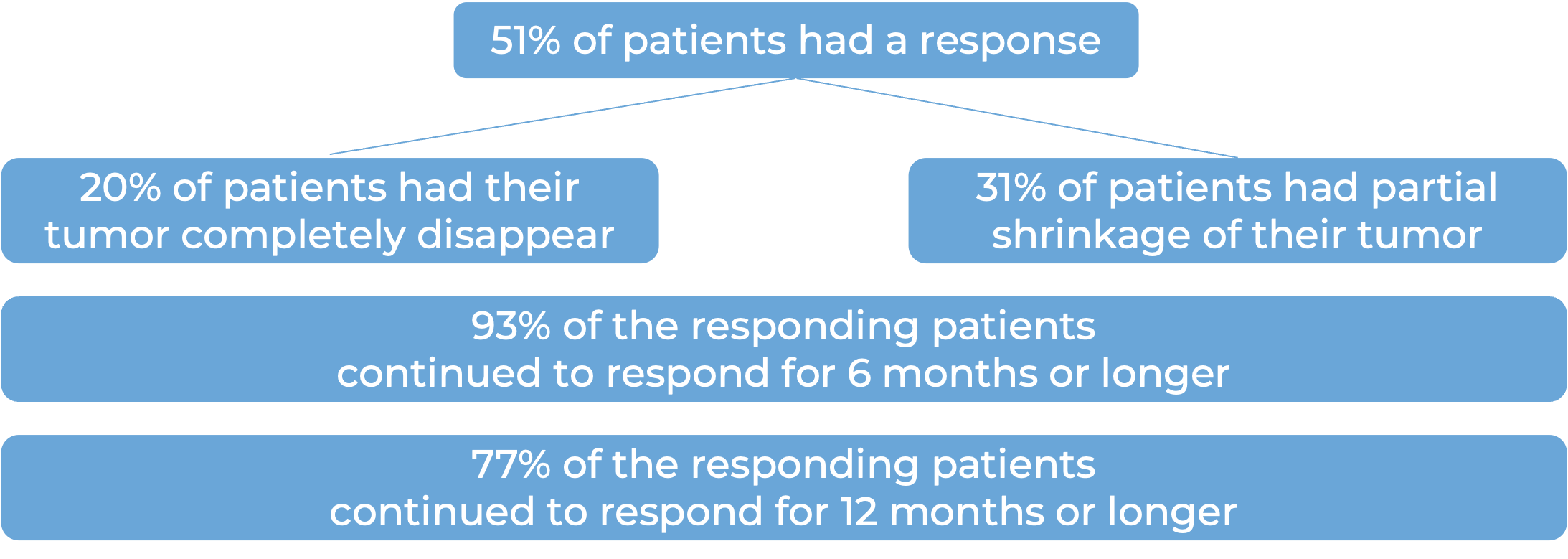
Among 56 patients with metastatic squamous cell skin cancer that had spread to other parts of the body who were treated with Libtayo every 3 weeks, at a median follow-up of 17 months:
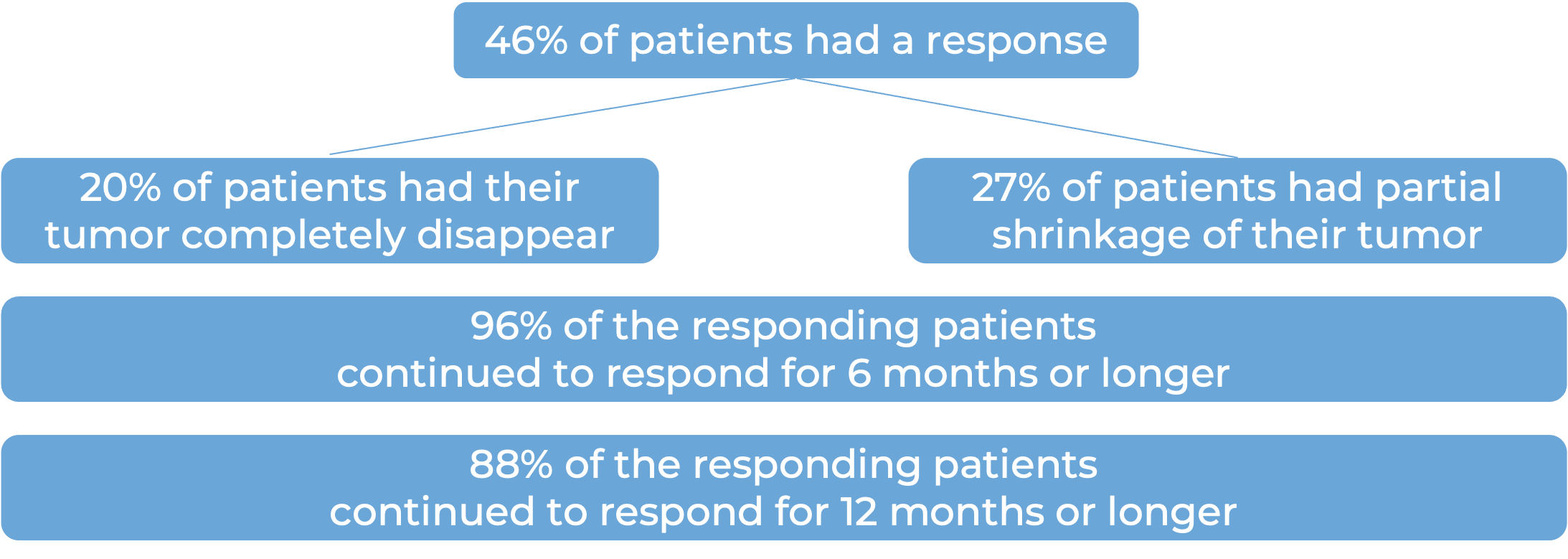
Basal cell carcinoma (BCC)
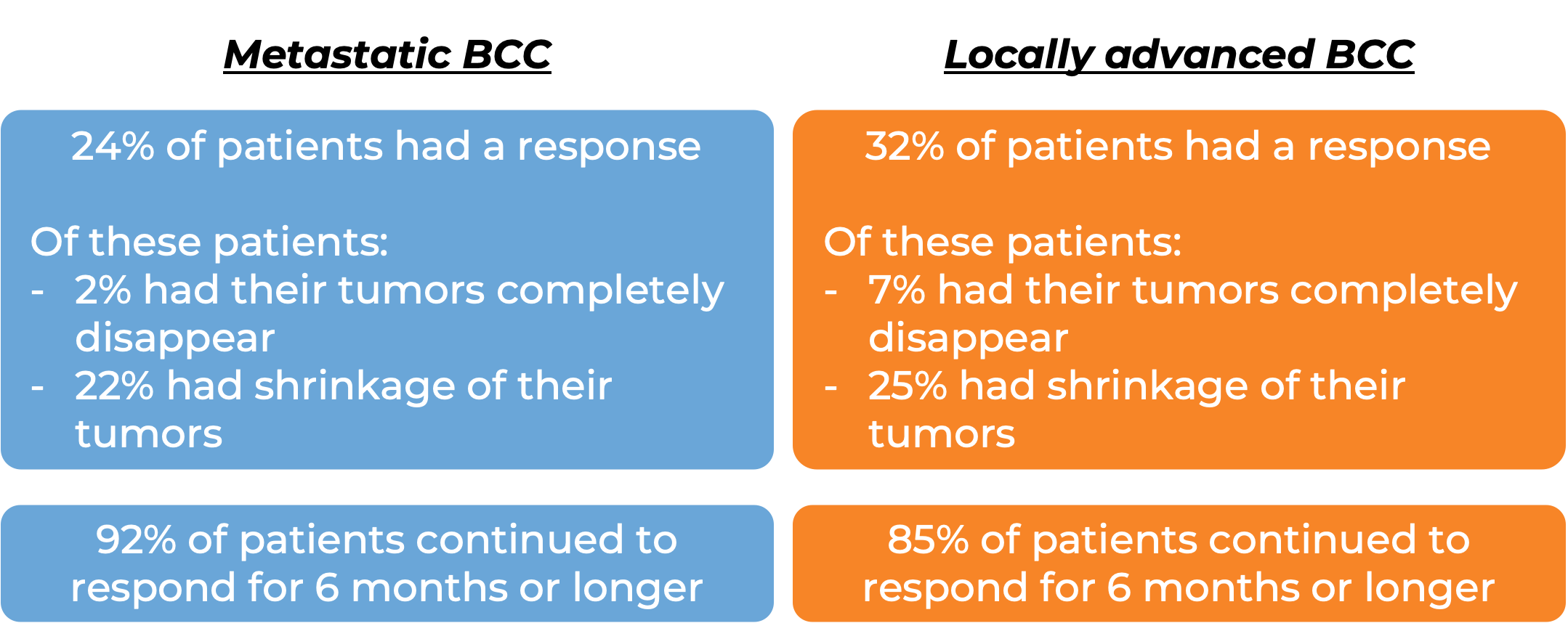
In a clinical trial, 138 patients with advanced basal cell carcinoma who were previously treated with a hedgehog pathway inhibitor (such as sonidegib [Odomzo] or vismodegib [Erivedge]) and it did not work, or who could not be treated with a hedgehog pathway inhibitor, were treated with Libtayo.
At a median follow-up of 8 months for patients whose cancer had spread to other parts of the body (metastatic) and 16 months for patients whose cancer had grown into nearby tissue (locally advanced), but had not spread to other parts of the body:
Non-small cell lung cancer
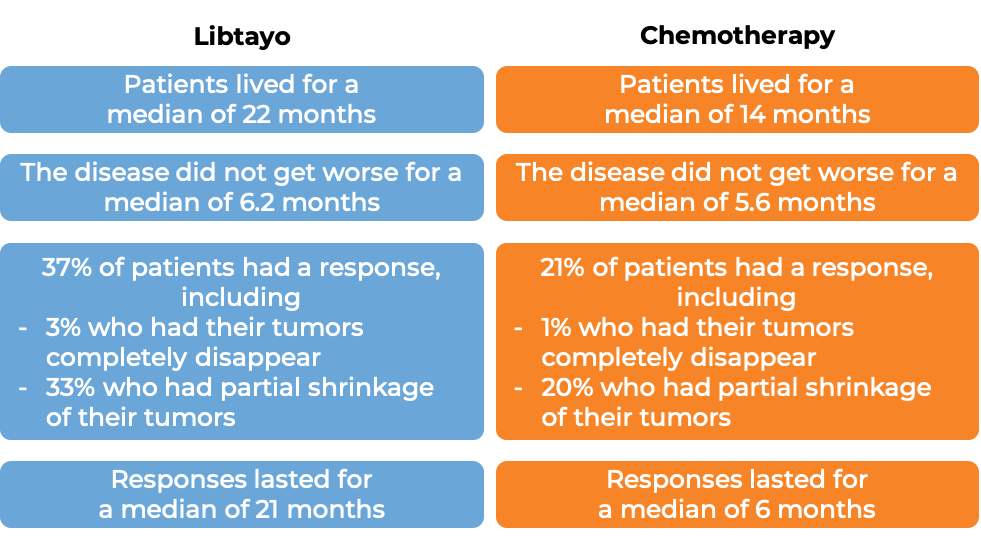
In a clinical trial, 710 patients with advanced non-small cell lung cancer that tested positive for high levels of the PD-L1 molecule, did not have an abnormal EGFR, ALK, or ROS1 gene, could not be treated with surgery or chemotherapy with radiation, or had spread outside the chest, and who had not received treatment for their advanced disease, were treated with either Libtayo or chemotherapy (platinum-based chemotherapy followed by optional pemetrexed therapy). At at median follow-up of 13 months:
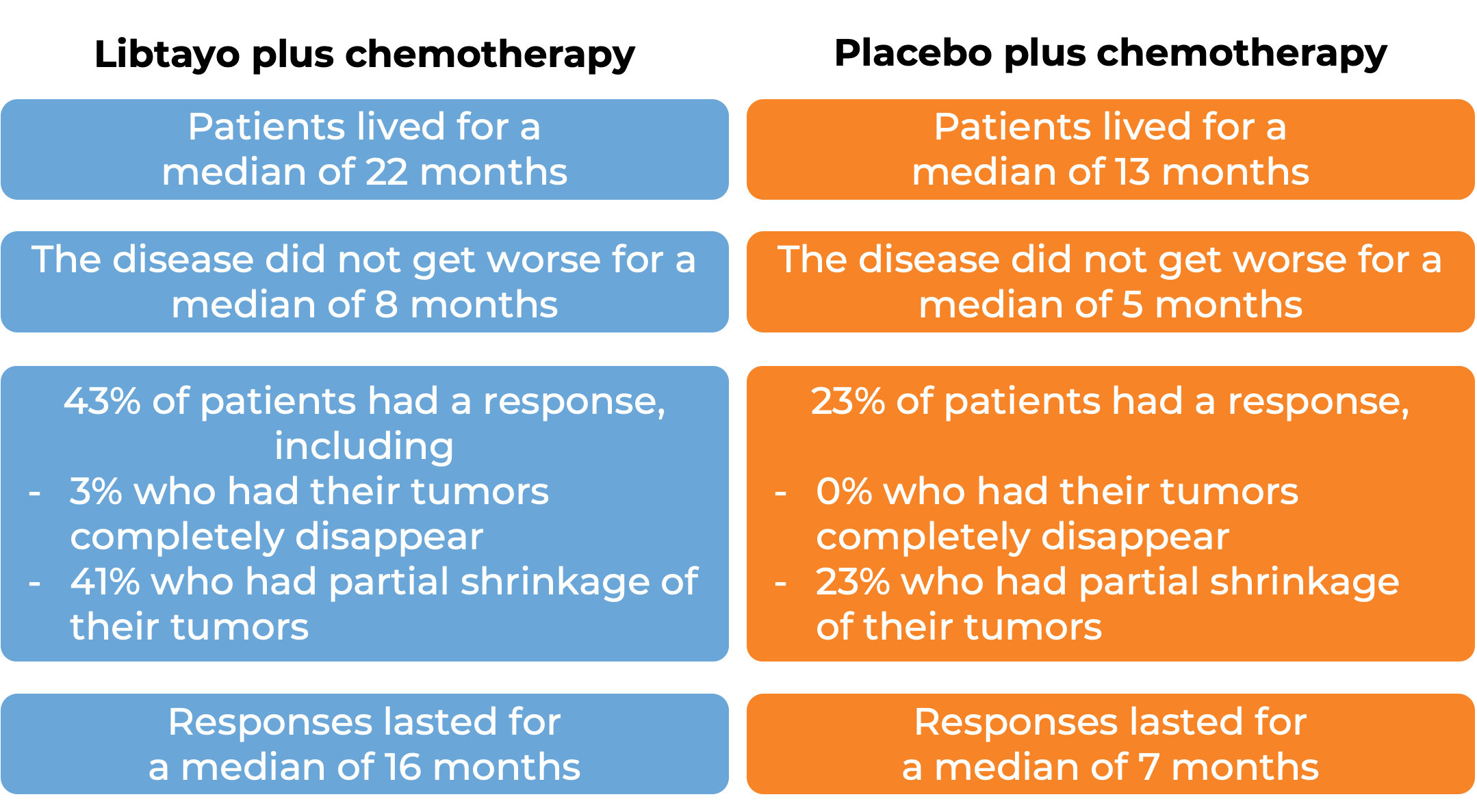
In a clinical trial, 466 patients with advanced non-small cell lung cancer that did not have an abnormal EGFR, ALK, or ROS1 gene, could not be treated with surgery or chemotherapy with radiation, or had spread outside the chest, and who had not received treatment for their advanced disease, were treated with either Libtayo and platinum-based chemotherapy or with placebo and platinum-based chemotherapy.
What are the side effects?
The most common side effects of Libtayo include tiredness, body aches, rash, itching, decreased appetite, diarrhea, pneumonia, and cough.
Libtayo can cause the patient’s T cells to attack healthy cells throughout the body. Because of this, Libtayo can cause side effects that can become serious or life-threatening, and may lead to death. Some of the serious side effects related to Libtayo include inflammation of the lungs, liver, brain, kidneys (which can lead to kidney failure), or colon (which can result in tears or holes in the intestine). Additionally, problems can arise with hormone glands (including thyroid, pituitary, and adrenal glands, as well as the pancreas). Libtayo may cause Type 1 diabetes. Skin rash (which could become severe and life-threatening), problems with the heart or eyes, and reactions related to the infusion may also occur.
Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects. For a more complete list of possible side effects, see the full prescribing information.
Manufacturer
Regeneron
Approval
FDA and EMA
Links to drug websites
Other references
Last updated on March 14, 2024
How is this drug name pronounced?
Generic name: seh-MIP-lih-mab
Brand name: lib-TY-oh
What cancer(s) does this drug treat?
Libtayo is approved for:
Cutaneous squamous cell carcinoma
Basal cell carcinoma
Non-small cell lung cancer
Cervical cancer
Cutaneous squamous cell carcinoma (CSCC)
Libtayo is approved for:
- Patients with squamous cell skin cancer that has grown or spread, and who cannot receive surgery or radiation.
Basal cell carcinoma
Libtayo is approved for:
- Patients with basal cell carcinoma that has grown into nearby tissue (locally advanced) OR has spread to other parts of the body (metastatic), and who have been previously treated with a hedgehog pathway inhibitor (such as sonidegib [Odomzo] or vismodegib [Erivedge]), or who cannot be treated with a hedgehog pathway inhibitor.
Non-small cell lung cancer
Libtayo is approved for:
Cervical cancer
Libtayo is approved for:
- Patients with cervical cancer that has come back or spread to other parts of the body and has gotten worse during or after treatment with platinum-based chemotherapy.
Limitations of use:
Age: The safety and efficacy of Libtayo in patients under 18 years of age have not been established.
Pregnancy/Breastfeeding: Libtayo can cause harm to a fetus, and is not recommended for use during pregnancy. The risks associated with Libtayo during breastfeeding are not known and cannot be ruled out; due to the potential for serious adverse reactions to the breastfed child, women are advised not to breastfeed during treatment and for at least 4 months after the last dose of Libtayo.
Complications of stem cell transplant: Serious transplant-related complications can occur in patients who have received a stem cell transplant from a stem cell donor before or after being treated with Libtayo.
Solid organ transplant rejection: Treatment with Libtayo can increase the risk of rejection in patients who have received an organ transplant.
What type of immunotherapy is this?
How does this drug work?
Libtayo is an antibody that attaches to a molecule called PD-1, which is present on the surface of T cells – the primary immune cells involved in killing cancer cells. In healthy tissues, PD-1 acts as a brake that keeps T cells from creating an immune reaction that gets out of control. However, cancers can hijack this safety mechanism and prevent T cells from doing their job – killing the cancer cells. This can happen when cancer cells or other cells within the tumor mass display on their surface molecules called PD-L1 or PD-L2. When PD-L1 or PD-L2 interact with PD-1 on T cells, the T cells become inactive and do not attack the cancer cells. Libtayo binds to the PD-1 molecules on T cells in such a way that prevents the interaction between PD-1 and PD-L1/PD-L2, and allows the T cells to be active and attack the cancer cells.

How is this drug given to the patient?
Libtayo is administered via a tube into a vein (intravenous infusion, or i.v.) over 30 minutes every three weeks and does not require a hospital stay.
What are the observed clinical results?
For:
Advanced cutaneous squamous cell carcinoma
Basal cell carcinoma
Advanced non-small cell lung cancer
Advanced cervical cancer
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Advanced cutaneous squamous cell carcinoma (CSCC)
In a clinical trial, 193 patients with advanced cutaneous squamous cell carcinoma who could not receive curative surgery or radiation, were treated with Libtayo.
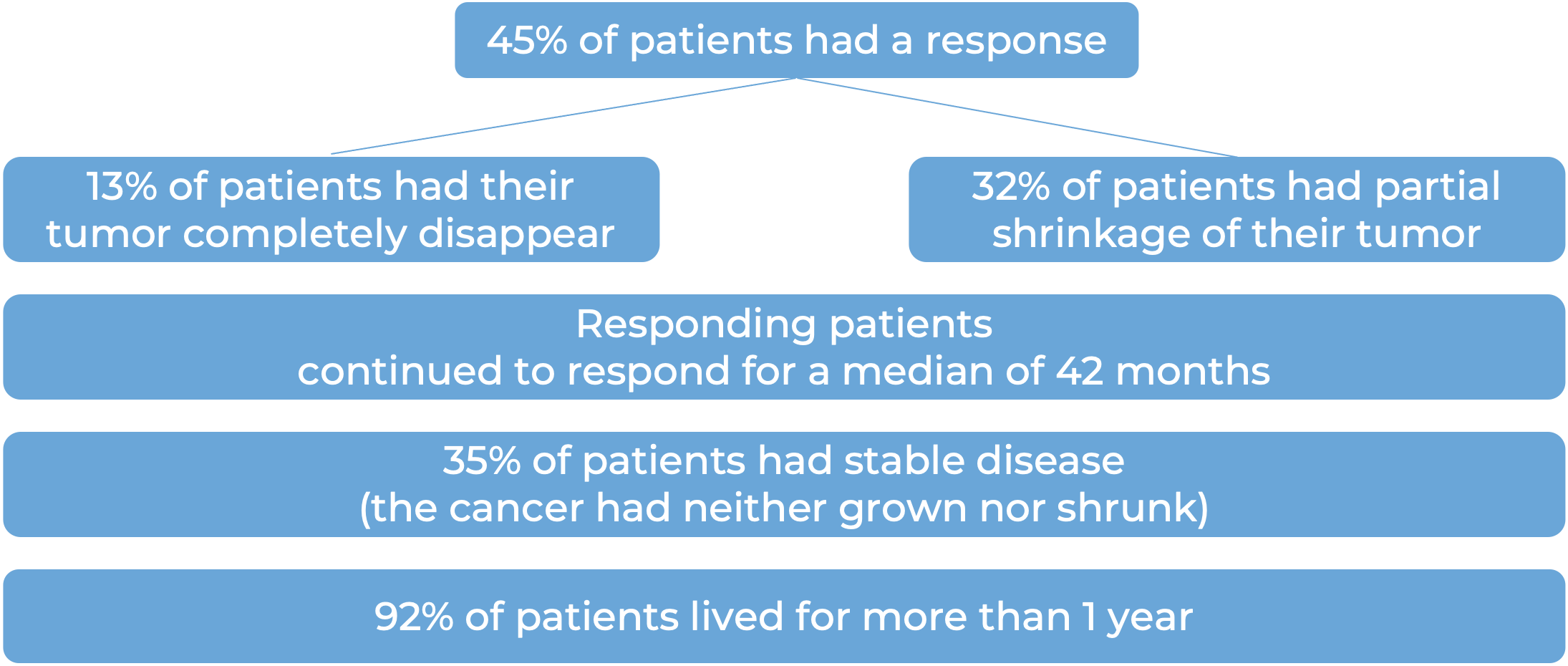
Among 78 patients with squamous cell skin cancer that had extensively grown at the initial tumor site, and who were treated with Libtayo every 2 weeks, at median follow-up of 16 months:
Among 59 patients with squamous cell skin cancer that had spread to other parts of the body and who were treated with Libtayo every 2 weeks, at median follow-up of 19 months:
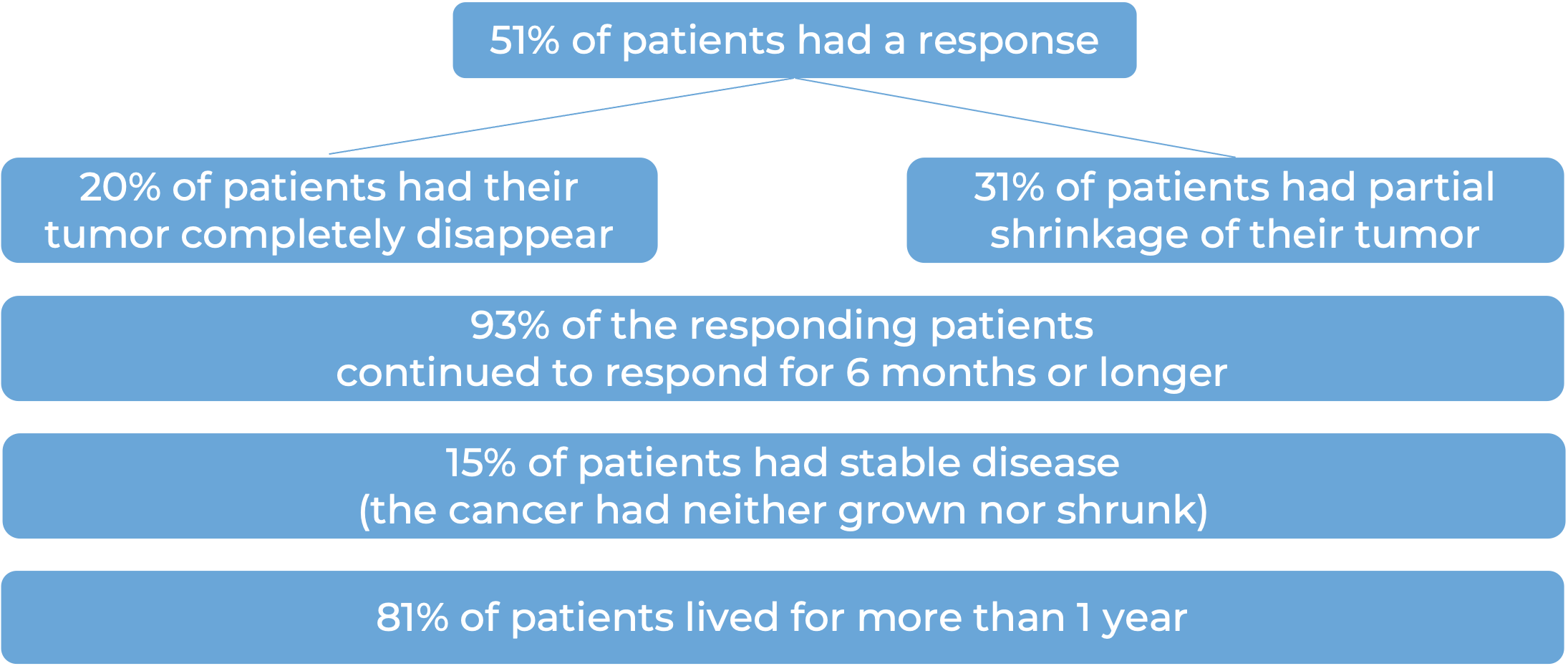
Among 56 patients with squamous cell skin cancer that had spread to other parts of the body and who were treated with Libtayo every 3 weeks, at median follow-up of 17 months:
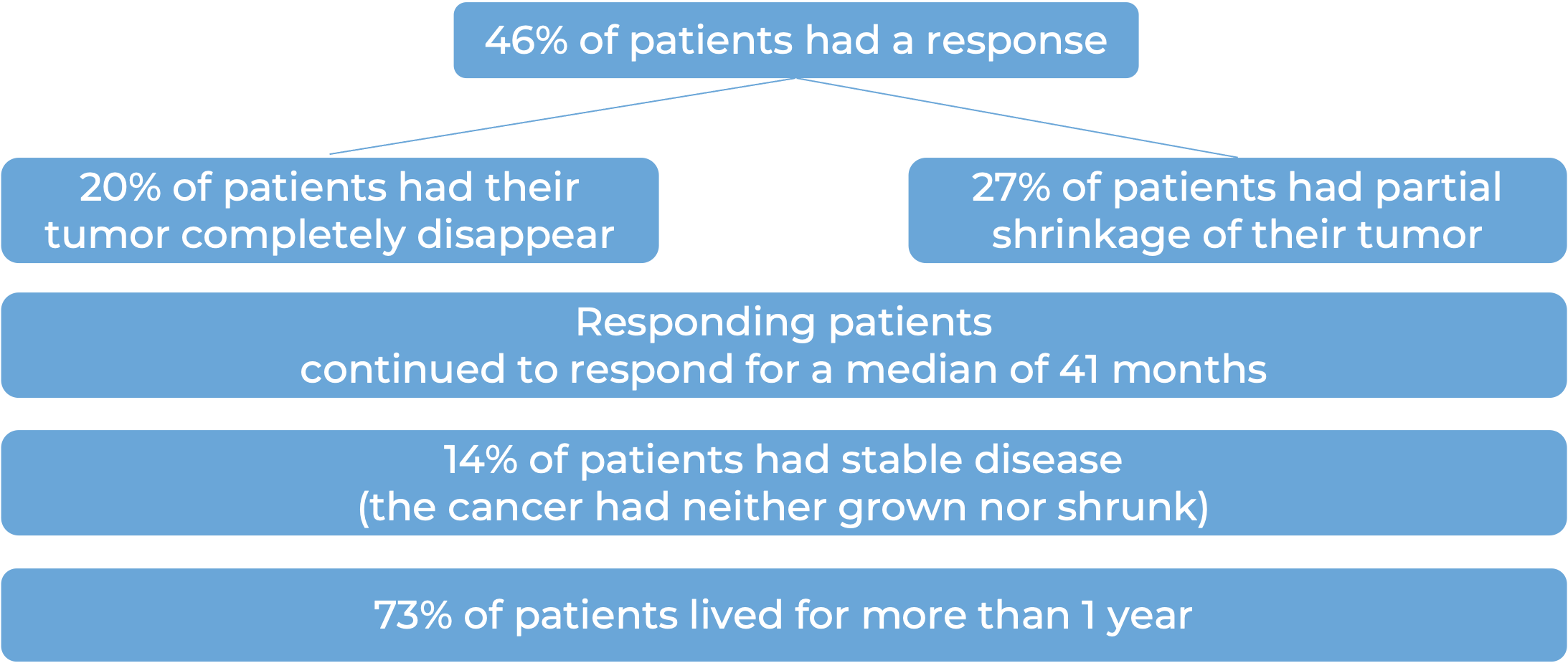
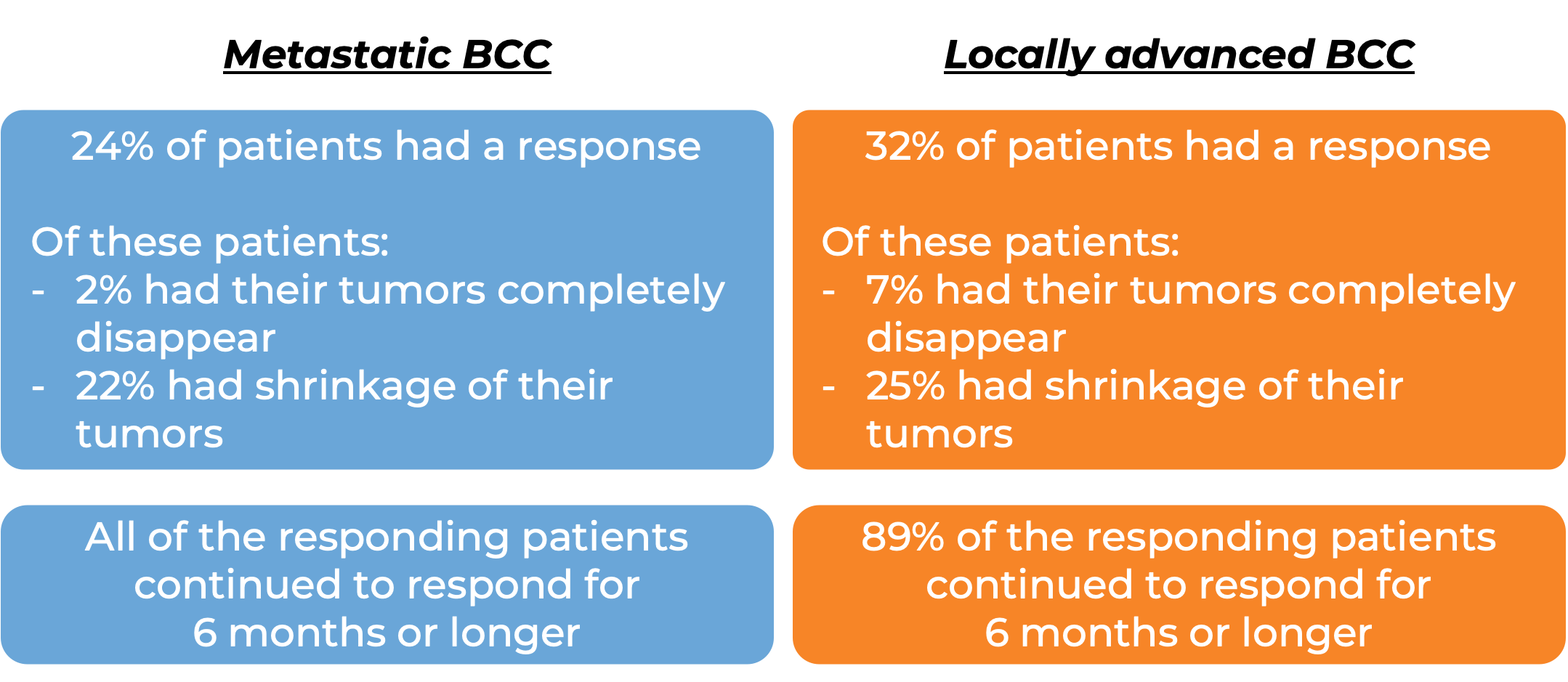
Basal cell carcinoma (BCC)
In a clinical trial, 138 patients with advanced basal cell carcinoma who were previously treated with a hedgehog pathway inhibitor (such as sonidegib [Odomzo] or vismodegib [Erivedge]) and it did not work, or who could not be treated with a hedgehog pathway inhibitor, were treated with Libtayo.
At a median follow-up of 8 months for patients whose cancer had spread to other parts of the body (metastatic), and 16 months for patients whose cancer had grown into nearby tissue (locally advanced), but had not spread to other parts of the body:
Advanced non-small cell lung cancer
In a clinical trial, 710 patients with advanced non-small cell lung cancer that tested positive for HIGH levels of the PD-L1 molecule, did not have an abnormal EGFR, ALK, or ROS1 gene, could not be treated with surgery or chemotherapy with radiation, or had spread outside the chest, and who had not received treatment for their advanced disease, were treated with either Libtayo or chemotherapy (platinum-based chemotherapy [followed by optional pemetrexed therapy]). At at median follow-up of 13 months:

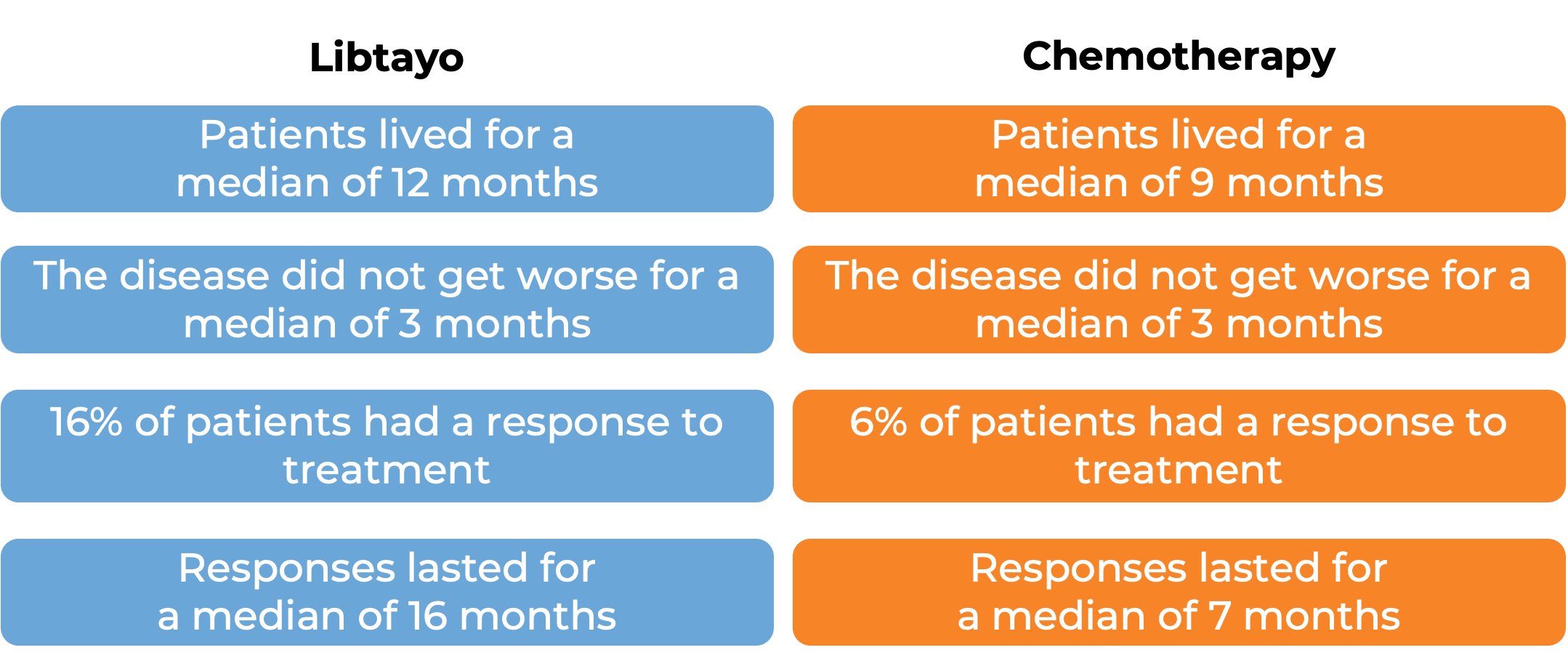
In another clinical trial, 466 patients with advanced non-small cell lung cancer that did not have an abnormal EGFR, ALK, or ROS1 gene, could not be treated with surgery or chemotherapy with radiation, or had spread outside the chest, and who had not received treatment for their advanced disease, were treated with either Libtayo plus chemotherapy or placebo plus chemotherapy. Among 327 patients who tested positive for LOW levels of PD-L1 and at a median follow-up of 16 months:
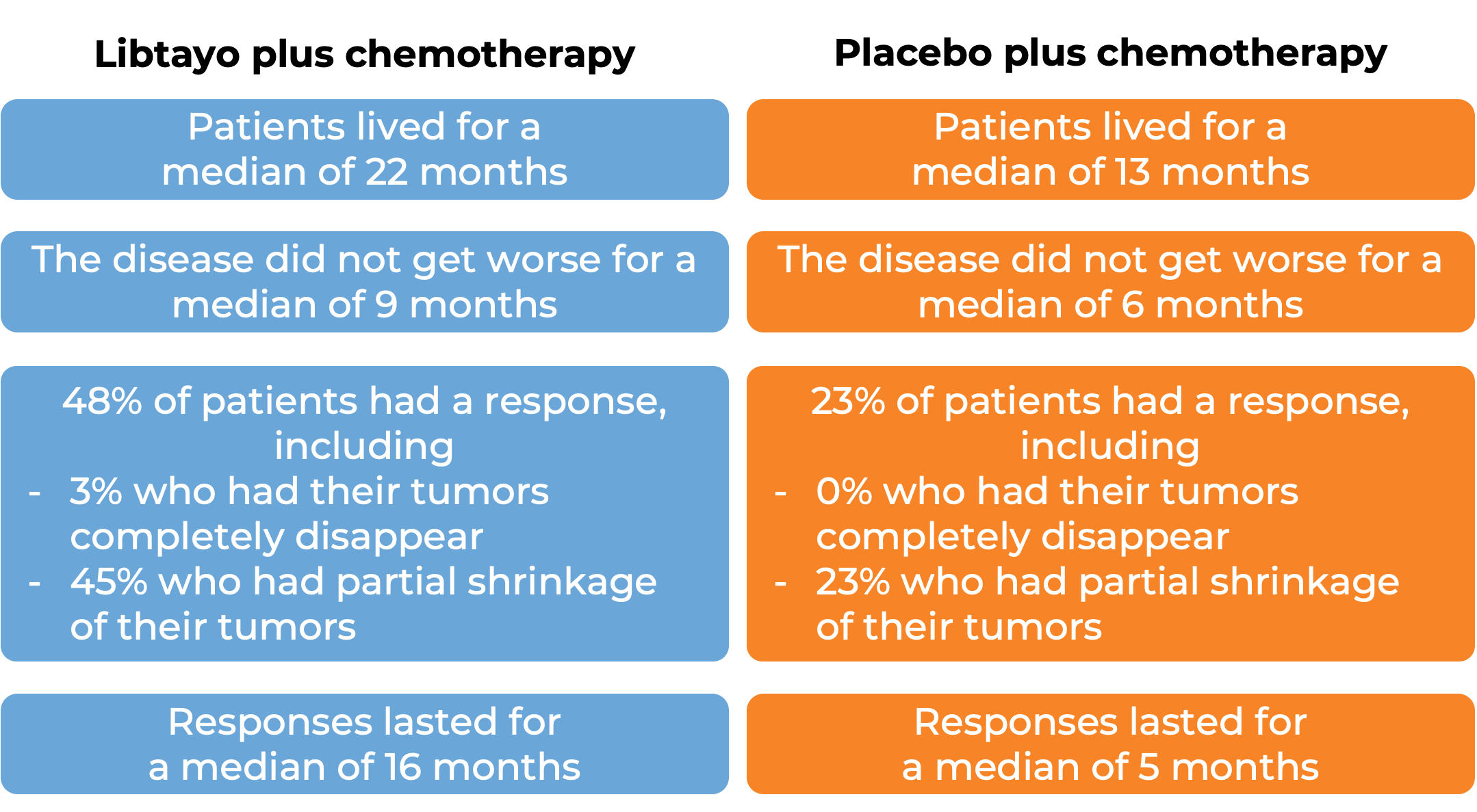
Advanced cervical cancer
In a clinical trial, 608 patients with advanced cervical cancer that came back or spread to other parts of the body and had gotten worse during or after treatment with platinum-based chemotherapy, were treated with Libtayo or chemotherapy (investigators choice of pemetrexed, topotecan, irinotecan, gemcitabine, or vinorelbine). At a median follow-up of 18 months:
What are the side effects?
The most common side effects of Libtayo include tiredness, nausea, loss of appetite, constipation, rash, itching, muscle or bone pain, abdominal pain, cough, upper respiratory tract infection, decreased red blood cells, and diarrhea.
Libtayo can cause the patient’s T cells to attack healthy cells throughout the body. Because of this, Libtayo can cause side effects that can become serious or life-threatening, and may lead to death. Some of the serious side effects related to Libtayo include inflammation of the lungs, liver, brain, kidneys (which can lead to kidney failure), or colon (that can result in tears or holes in the intestine). Additionally, problems can arise with hormone glands (including thyroid, pituitary, and adrenal glands, as well as the pancreas), skin rash (which could become severe and life-threatening), hemophagocytic lymphohistiocytosis (HLH; an excessive immune activation), and reactions related to the infusion. Libtayo can cause Type 1 diabetes. Patients should report any symptoms to their healthcare provider who can then initiate actions to limit or reverse the side effects.
For a more complete list of possible side effects, see the full prescribing information.
Manufacturer
Regeneron
Approval
FDA and EMA
Links to drug websites
Last updated on March 14, 2024















