How is this drug name pronounced?
Ipilimumab:ih-pih-LIH-myoo-mab
Yervoy: YER-voy
What cancer(s) does this drug treat?
Yervoy is approved for:
Melanoma
Kidney cancer
Colorectal cancer (MSI-H/dMMR)
Liver cancer
Non-small cell lung cancer
Mesothelioma
Esophageal cancer
Advanced melanoma
Yervoy is approved for:
- Adult and pediatric patients 12 years of age and older with advanced melanoma that is metastatic (cancer has spread to other parts of the body from the original cancer site) or cannot be completely removed by surgery. Pediatric patients are treated with Yervoy alone, while adult patients may be treated with Yervoy alone or in combination with nivolumab (Opdivo).
- Adult patients with skin melanoma that has spread to nearby lymph nodes and has been completely removed by surgery. In such cases, Yervoy is used to help keep melanoma from coming back.
Advanced kidney cancer
Yervoy is approved for:
- Adult patients with previously untreated advanced renal cell carcinoma (kidney cancer) who are at moderate or high risk of their cancer getting worse. In such cases, patients are treated with Yervoy in combination with nivolumab (Opdivo).
Advanced colorectal cancer (MSI-H/dMMR){#advanced-colorectal-cancer}
Yervoy is approved for:
- Adult and pediatric patients 12 years of age and older with colon or rectal cancer that has spread, have a tumor that is microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR), and have been treated with a fluoropyrimidine, oxaliplatin, and irinotecan, and the treatment did not work or stopped working. In such cases, patients are treated with Yervoy in combination with nivolumab (Opdivo).
Advanced liver cancer
Yervoy is approved for:
- Adult patients with hepatocellular carcinoma (liver cancer) who have been treated with sorafenib (Nexavar). In such cases, patients are treated with Yervoy in combination with nivolumab (Opdivo).
Advanced non-small cell lung cancer
Yervoy is approved for:
- Adult patients with non-small cell lung cancer that has spread, and whose tumors test positive for the PD-L1 molecule and do not have an abnormal EGFR or ALK gene. In such cases, Yervoy is used in combination with nivolumab (Opdivo) as a first treatment.
- Adult patients with non-small cell lung cancer that has spread or come back, whose tumors do not have an abnormal EGFR or ALK gene. In such cases, Yervoy is used in combination with nivolumab (Opdivo) and two treatment cycles of platinum-containing chemotherapy as a first treatment.
Mesothelioma
Yervoy is approved for:
- Adult patients with newly diagnosed malignant pleural mesothelioma (a cancer in the cells that line the inside of the chest and cover the lungs) whose cancer cannot be removed by surgery may be treated with Yervoy in combination with nivolumab (Opdivo).
Advanced esophageal cancer
Yervoy is approved for:
- Adult patients with esophageal squamous cell carcinoma that has grown and spread to nearby lymph nodes and cannot be removed by surgery, or has spread to other parts of the body. In such cases, Yervoy may be used in combination with nivolumab (Opdivo) as a first treatment for their advanced disease.
Limitations of use:
Age: The safety and efficacy of Yervoy in patients under 12 years of age or under 18 years of age, as indicated for individual cancer types and settings, have not been established.
Pregnancy/Breastfeeding: Yervoy can cause harm to a fetus, and is not recommended for use during pregnancy. Women who are able to become pregnant should use effective birth control during treatment and for at least 3 months after the last dose of Yervoy. The risks associated with Yervoy during breastfeeding are not known and cannot be ruled out; women are advised not to breastfeed during treatment and for 3 months after the last dose of Yervoy.
Complications of stem cell transplant: Serious and life-threatening complications, which can lead to death, can occur in patients who have received a stem cell transplant from a stem cell donor before or after being treated with Yervoy.
What type of immunotherapy is this?
- CTLA-4 blockade
How does this drug work?
- Target: CTLA-4
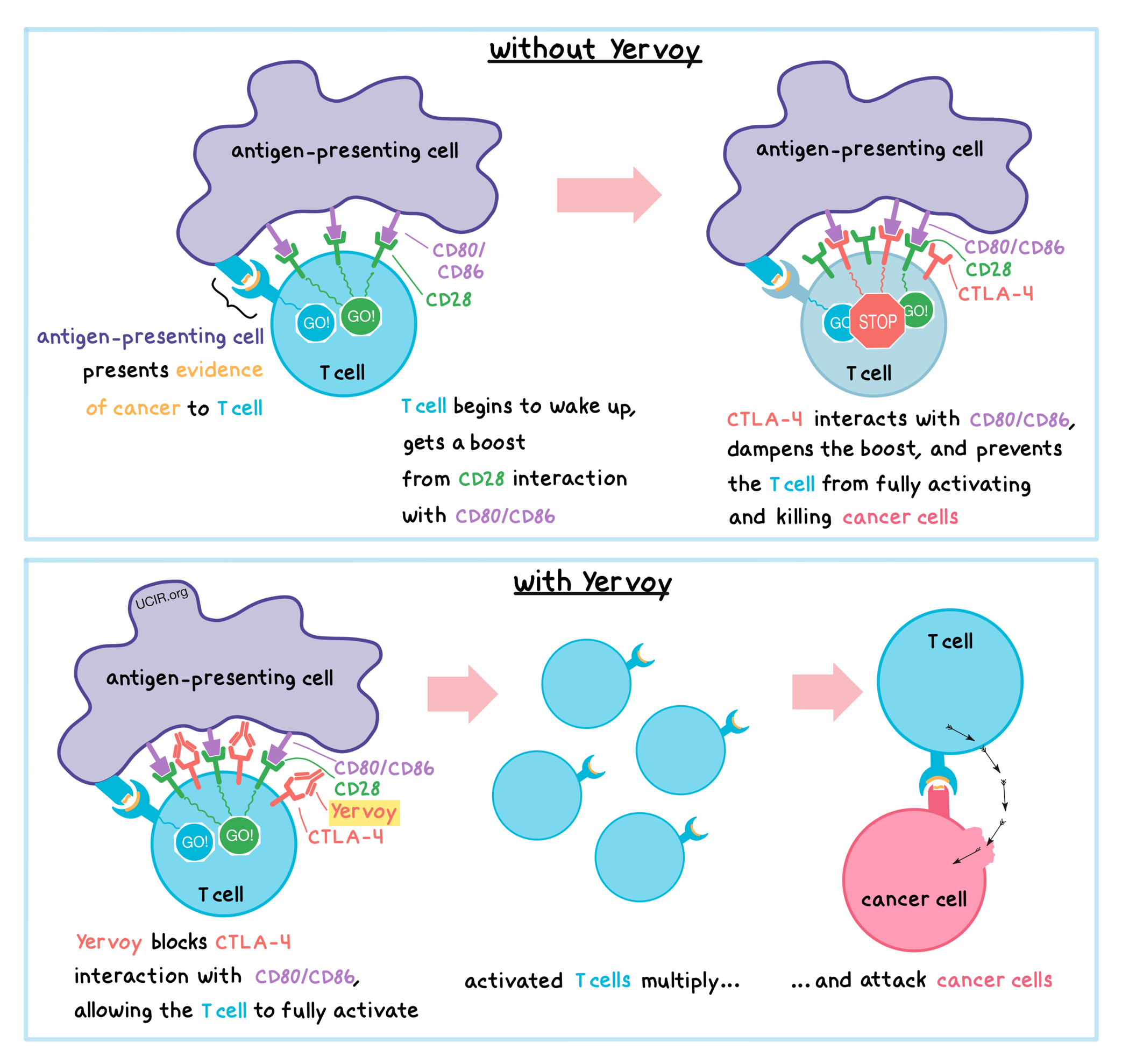
Yervoy is an antibody that attaches to a molecule called CTLA-4, which is present on the surface of T cells – the primary immune cells involved in the killing of cancer cells.
Before T cells can kill cancer cells, they first need to be “woken up”, or activated, and then they have to multiply to launch a potent attack. To help activate T cells, vigilant antigen-presenting cells patrol the body, pick up evidence of cancer, and travel to the lymph nodes to present this evidence to the T cells. If a particular T cell recognizes the evidence of cancer, it begins to wake up. However, to be fully activated for the cancer battle, T cells need a “caffeine” boost. This boost occurs when an activating molecule called CD28 on the surface of T cells interacts with molecules called CD80 or CD86 on the surface of antigen-presenting cells. However, shortly after the boost occurs, CTLA-4 shows up on the surface of T cells and competes with CD28 for the attention of CD80 or CD86, keeping the T cells from getting too much of the “caffeine” boost. Thus, CTLA-4 acts as a brake that keeps the T cells from creating an immune reaction that gets out of control, which could damage healthy tissues. Unfortunately, in cancer, the interaction between CTLA-4 and CD80/CD86 can prevent T cells from becoming activated enough to kill the cancer cells.
Yervoy binds to the CTLA-4 molecules on T cells in such a way that blocks the interaction between CTLA-4 and CD80/CD86, and allows the CD28 molecules on T cells to keep interacting with CD80/CD86 to fully activate the T cells, which is needed to attack cancer. Once the T cells are fully activated, they multiply to create a large army of potent T cells that can hunt down cancer cells throughout the body and kill them wherever they are found.
There may be other ways in which Yervoy helps destroy the cancer, and they are currently under investigation.
How is this drug given to the patient?
Yervoy is administered via a tube into a vein (intravenous infusion, or i.v.) over 30 or 90 minutes (depending on the dose and the type of cancer being treated). Yervoy is usually administered every three weeks for the first four doses when given alone, and every 6 weeks in combination with nivolumab (Opdivo) for the treatment of mesothelioma, non-small cell lung cancer, or esophageal cancer. The frequency of subsequent dosing and the maximum number of doses depends on the cancer type. When given in combination with nivolumab (Opdivo), administration of Yervoy is immediately followed by nivolumab (Opdivo) on the same day, and the combination treatment is given every three or six weeks. Administration of Yervoy does not require a hospital stay.
What are the observed clinical results?
For:
Advanced melanoma (metastatic or not removable by surgery)
Advanced melanoma (completely removed by surgery)
Advanced kidney cancer (previously untreated)
Advanced colorectal cancer (MSI-H/dMMR)
Advanced liver cancer
Advanced non-small cell lung cancer
Malignant pleural mesothelioma
Advanced esophageal cancer
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Advanced melanoma (metastatic or not removable by surgery)
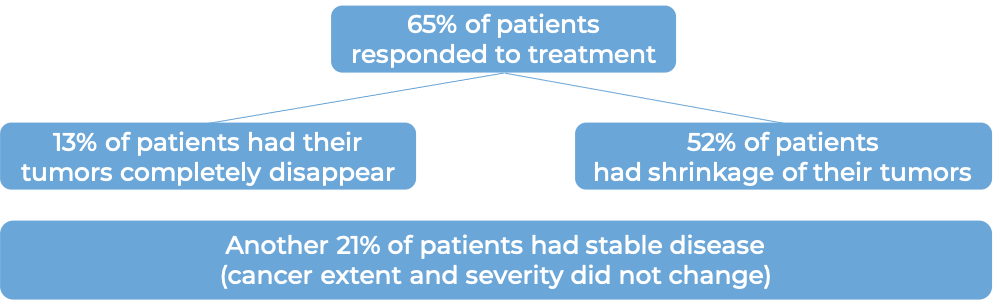
In a clinical trial, 676 patients with previously treated melanoma that had spread to other parts of the body or could not be removed by surgery, were treated with either Yervoy, an experimental cancer vaccine (gp100), or a combination of Yervoy and gp100. At a median follow-up of 9 months:

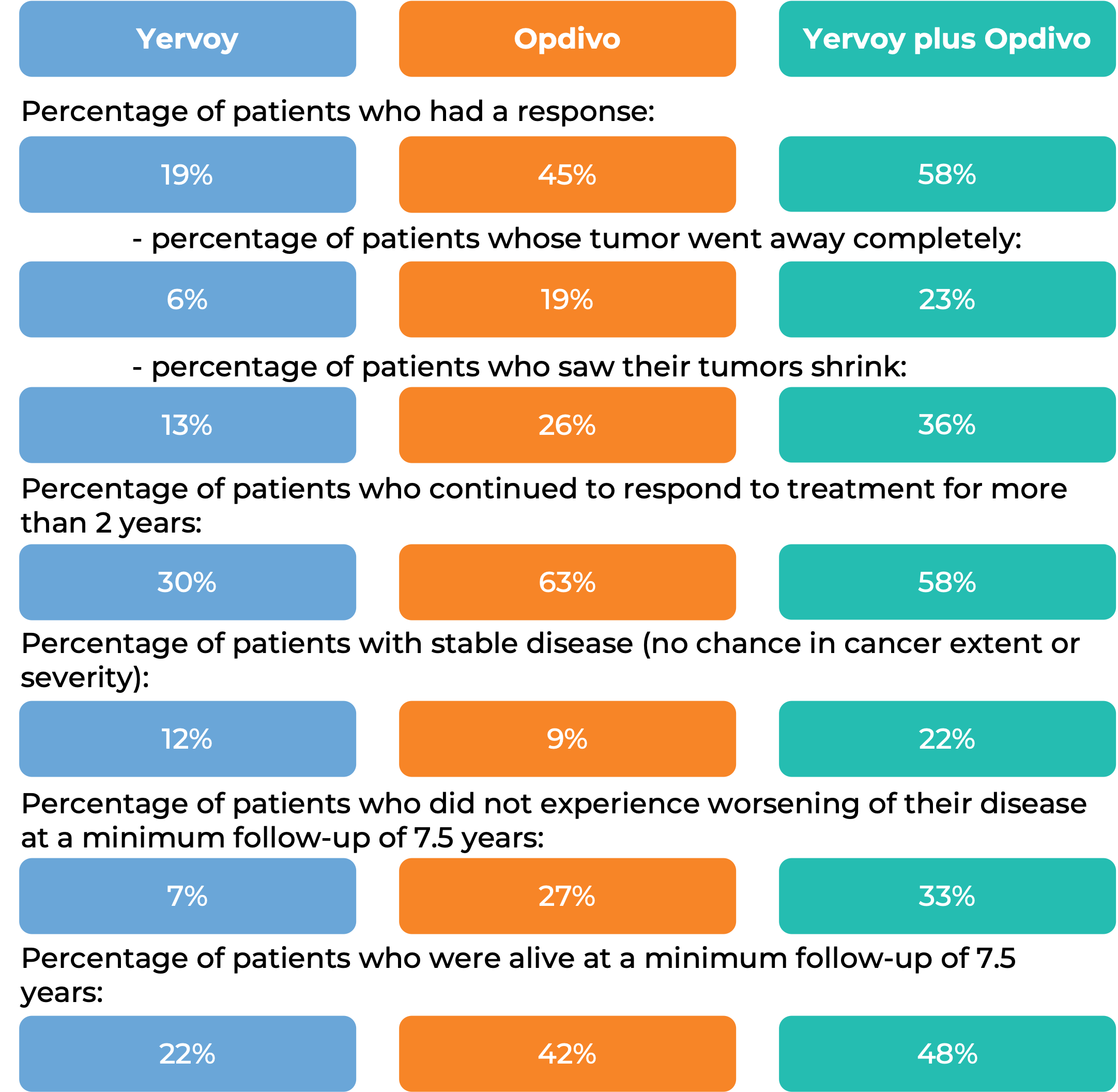
In another clinical trial, 945 patients with previously untreated melanoma that had spread to other parts of the body or could not be removed by surgery, were treated with either Yervoy in combination with nivolumab (Opdivo), Opdivo alone, or Yervoy alone.
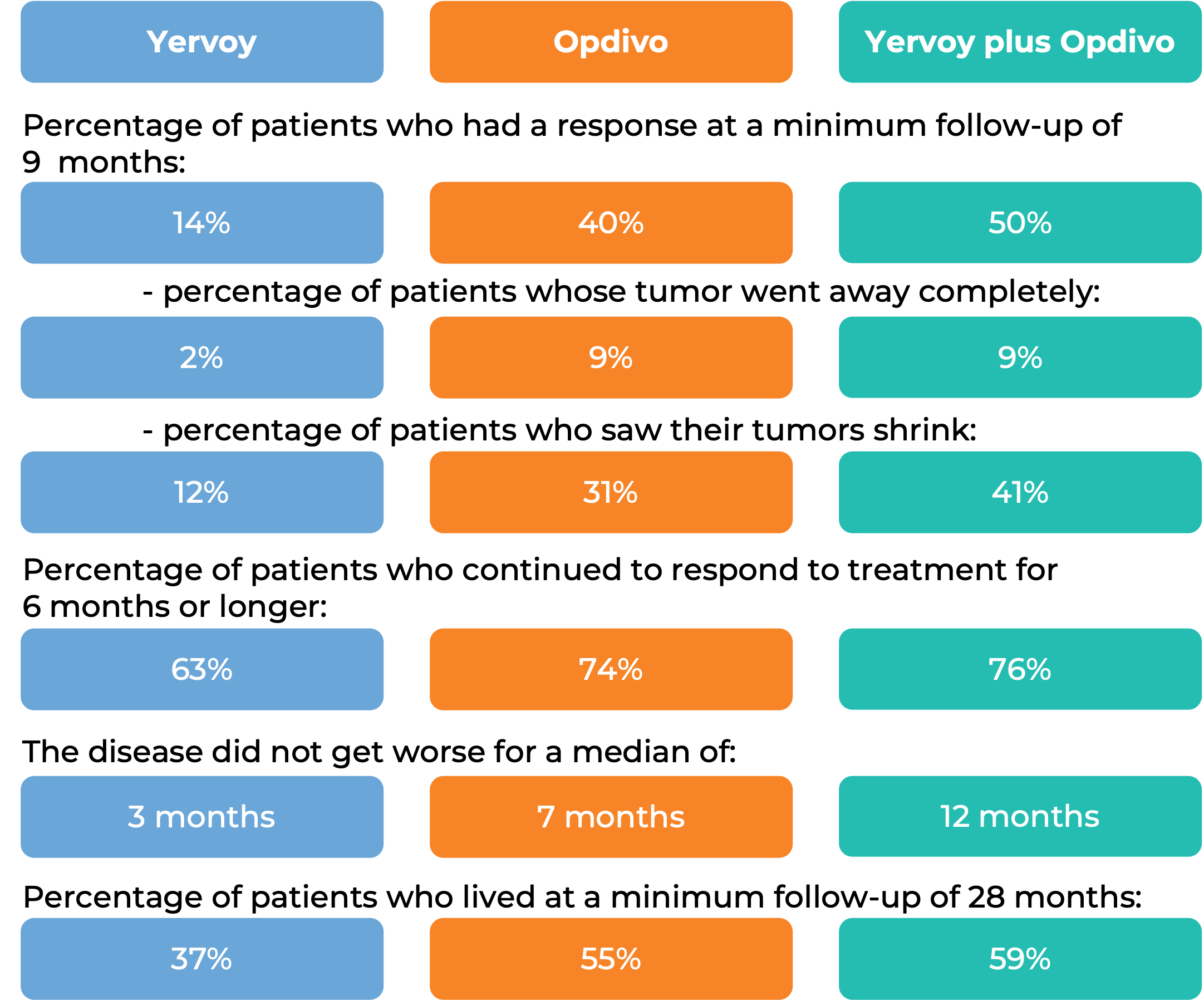
(For a definition of median click here.)
Advanced melanoma (completely removed by surgery)
In a clinical study, 951 patients with melanoma that was completely removed by surgery were treated with either Yervoy or placebo. At a median follow-up of 5.3 years:
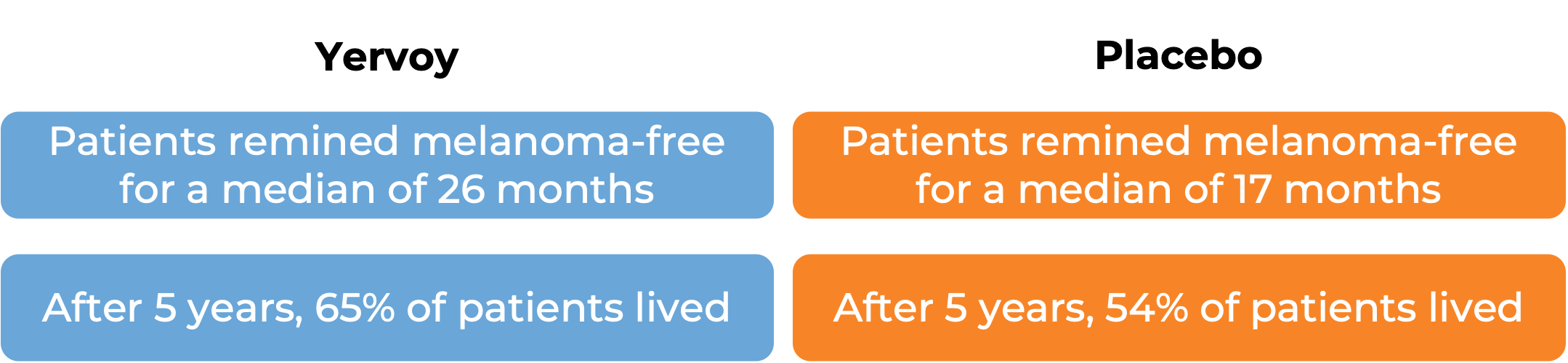
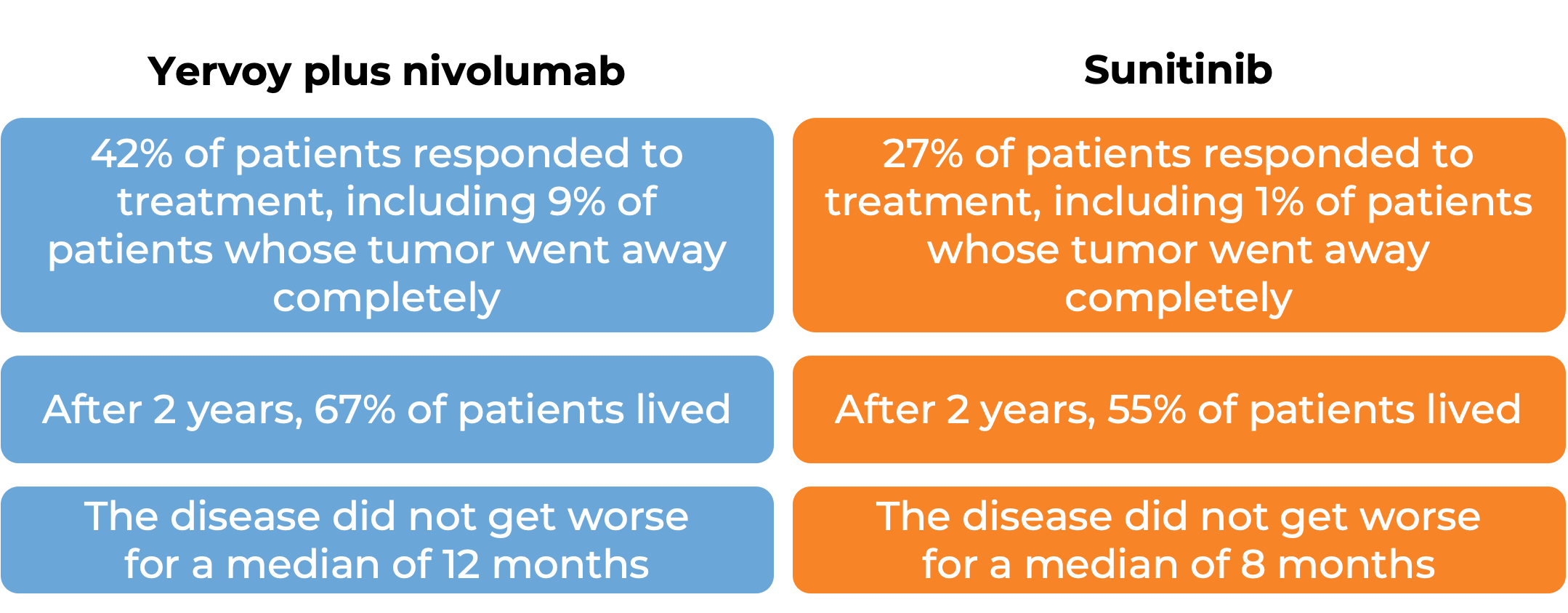
Advanced kidney cancer (previously untreated)
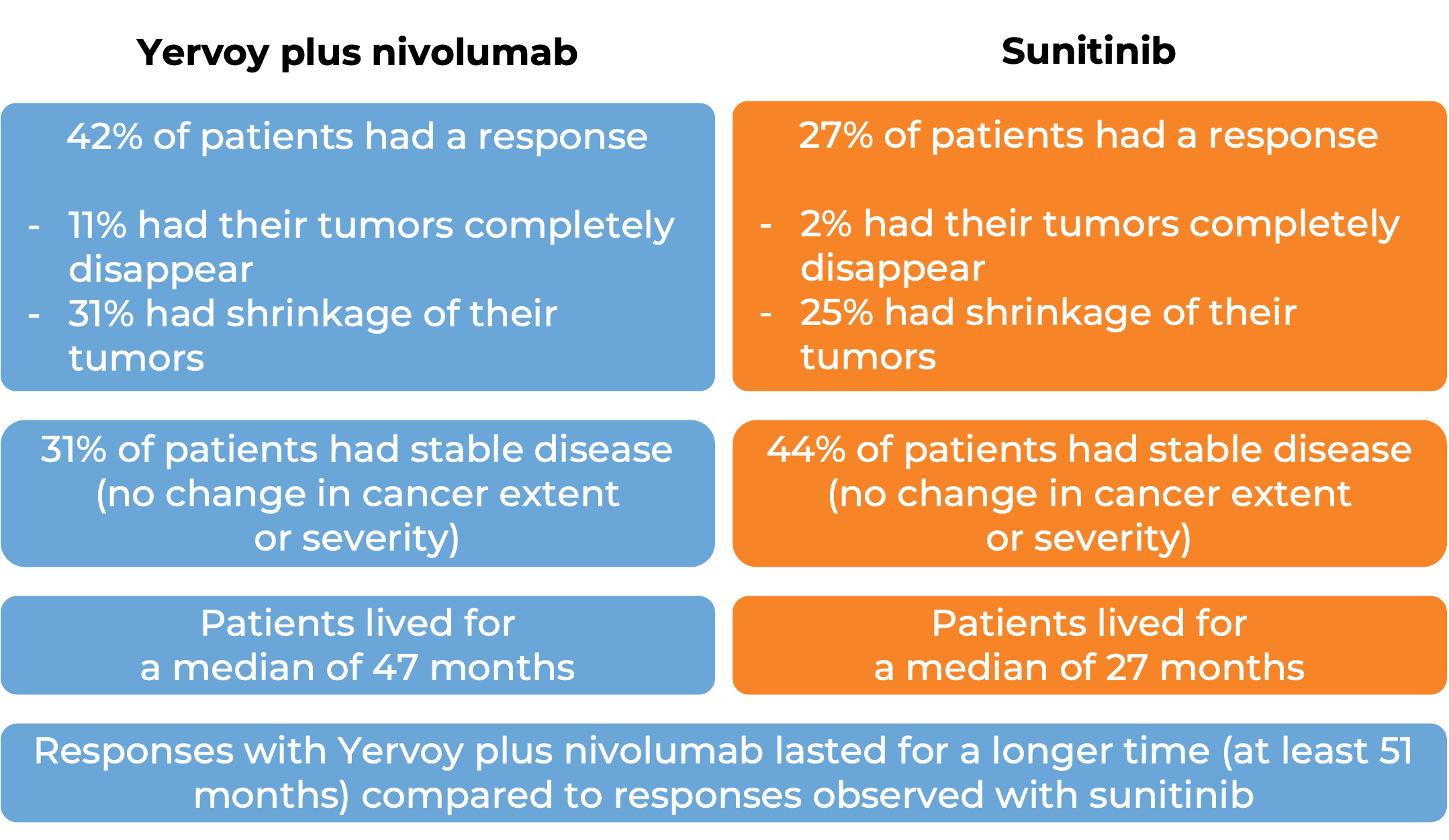
In a clinical study, 847 patients with previously untreated advanced kidney cancer were treated with either a combination of Yervoy and nivolumab (Opdivo) or with sunitinib. At a median follow-up of 15 months:
Advanced colorectal cancer (MSI-H/dMMR)
In a clinical study, 119 patients with microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) colon or rectal (“colorectal”) cancer were treated with a combination of Yervoy and nivolumab (Opdivo). Among 82 patients who had been previously treated with a fluoropyrimidine, oxaliplatin, and irinotecan, and the treatment did not work or stopped working, at a minimum follow-up of 28 months:
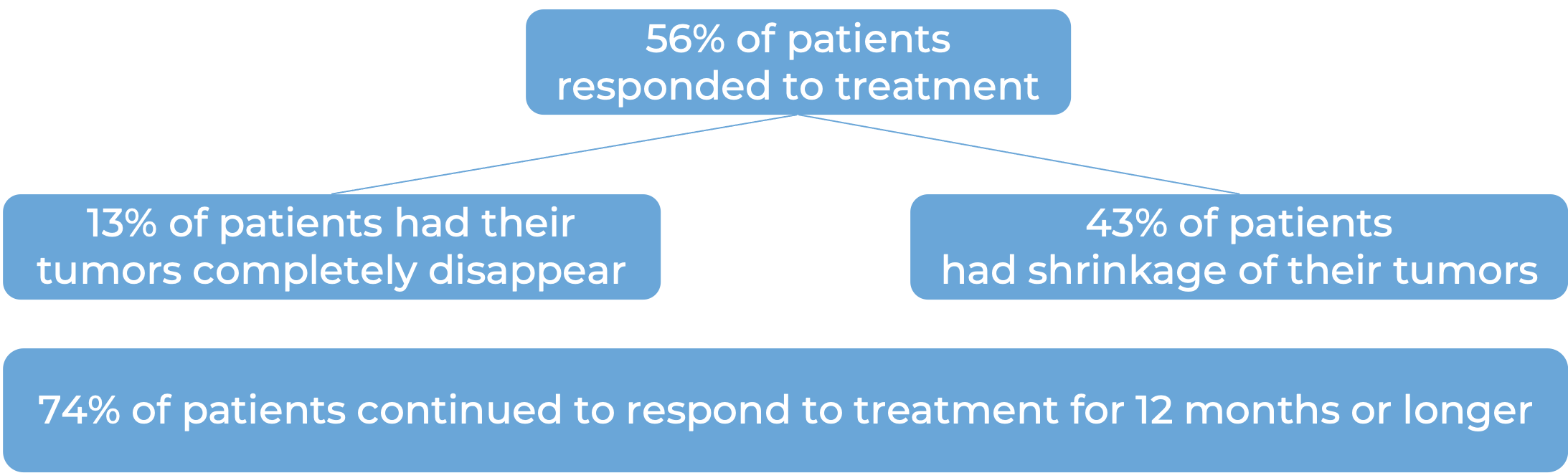
Advanced liver cancer
In a clinical trial, 49 patients with advanced liver cancer who had been treated with sorafenib (Nexavar), and it either did not work, stopped working, or was not tolerated, were treated with Yervoy in combination with nivolumab (Opdivo). At a minimum follow-up of 28 months:
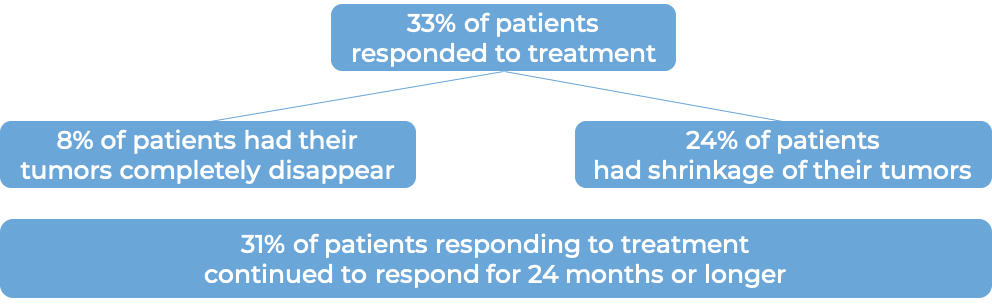
Advanced non-small cell lung cancer
In a clinical study of patients with previously untreated non-small cell lung cancer that had spread or come back, and whose tumors did not have an abnormal EGFR or ALK gene, were treated with either Yervoy plus nivolumab (Opdivo) or platinum-containing chemotherapy. At a minimum follow-up of 29 months, among the 793 patients whose tumors tested positive for the PD-L1 molecule:
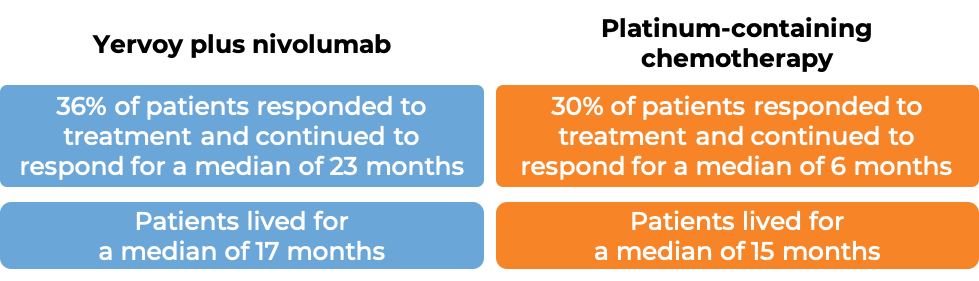
(For a definition of median click here.)
In another clinical study of 719 patients with non-small cell lung cancer that had spread or come back, whose tumors did not have an abnormal EGFR or ALK gene, and who had not received treatment for their advanced disease, were treated with either:
- Yervoy, nivolumab, and platinum-containing chemotherapy, OR
- platinum-containing chemotherapy.
At a minimum follow-up of 13 months:
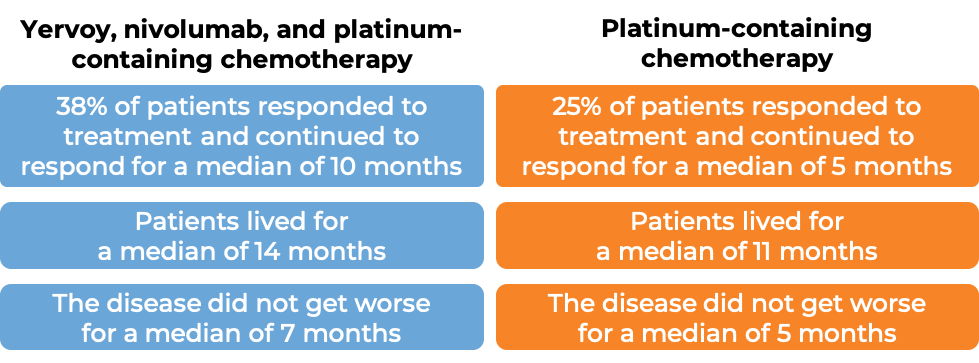
(For a definition of median click here.)
Malignant pleural mesothelioma
In a clinical study of 605 patients with newly diagnosed malignant pleural mesothelioma whose cancer could not be removed by surgery, patients were treated with either Yervoy in combination with nivolumab (Opdivo) for up to 2 years, or chemotherapy (cisplatin or carboplatin plus pemetrexed). At a minimum follow-up of 22 months:
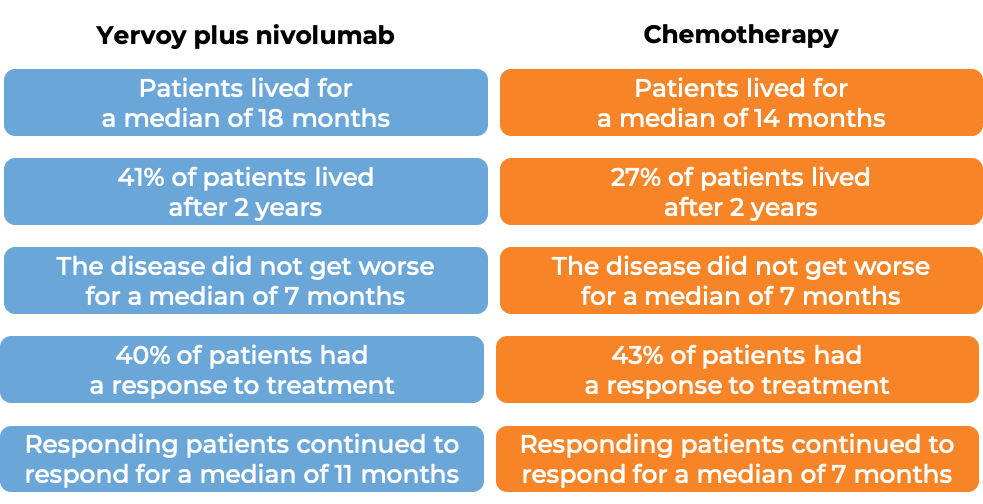
(For a definition of median click here.)
Advanced esophageal cancer
In a clinical trial, 649 patients with esophageal squamous cell carcinoma whose cancer had come back, spread to nearby lymph nodes and could not be removed by surgery or had spread to other parts of the body, were treated with either Yervoy plus nivolumab (Opdivo) or chemotherapy (cisplatin and fluorouracil). At a minimum follow-up of 13 months:
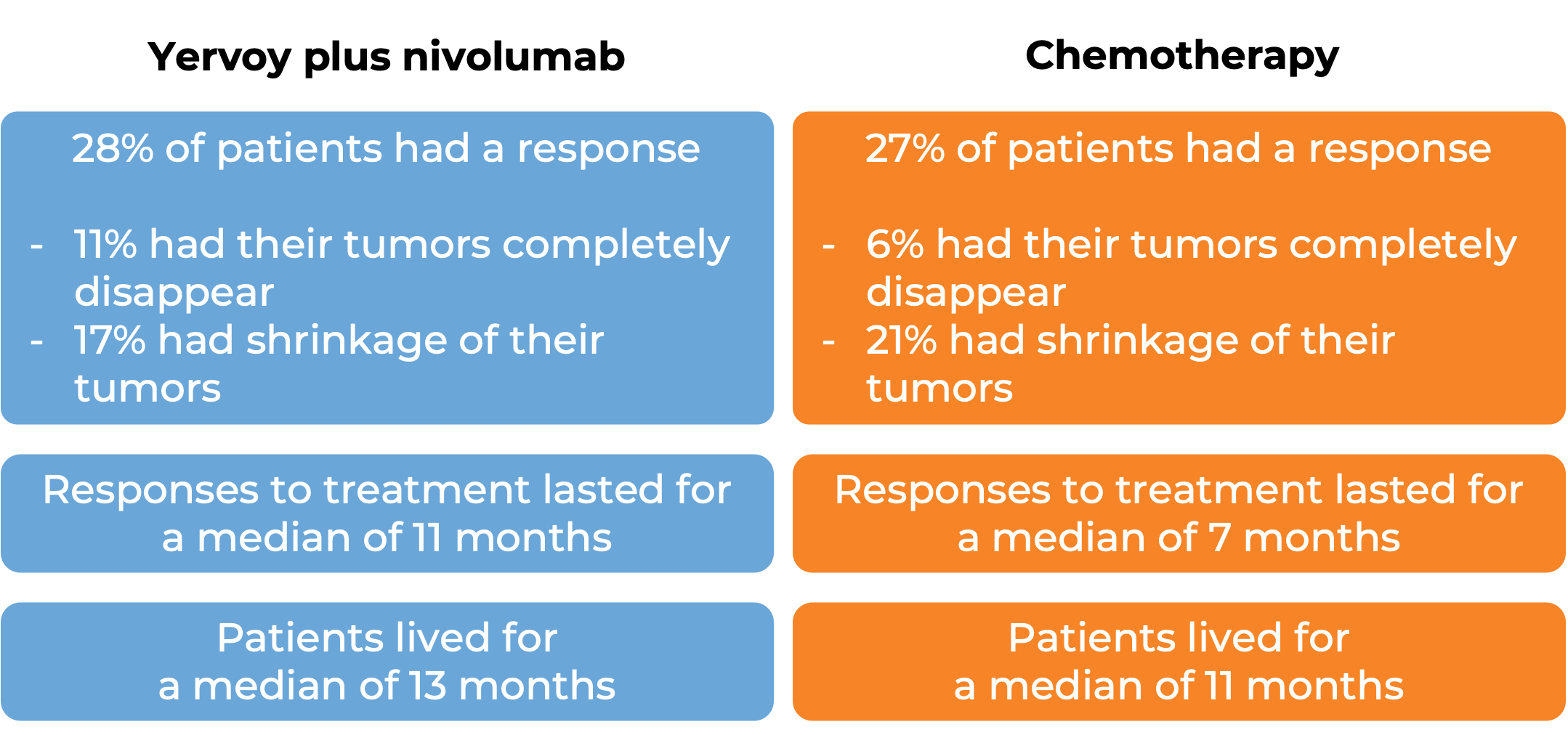
Among 315 patients whose cancer tested positive for the PD-L1 molecule:
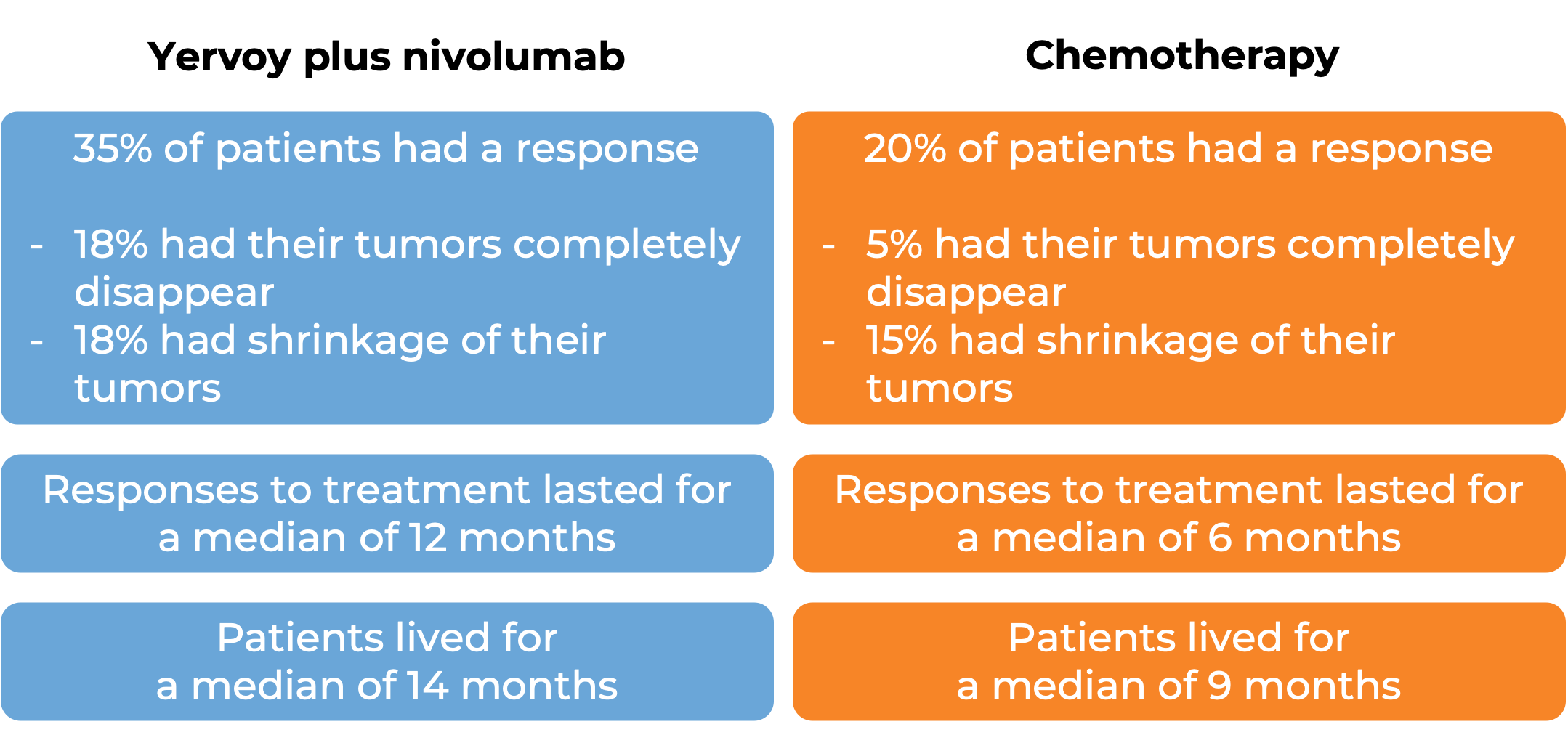
(For a definition of median click here.)
What are the side effects?
The most common side effects of Yervoy include fatigue, diarrhea, nausea, vomiting, decreased appetite, weight loss, itching, rash, headache, fever, and difficulty falling asleep or staying asleep. The most common side effects of Yervoy when used in combination with nivolumab (Opdivo) (in addition to those mentioned before) include belly ache, pain in muscles, bones, and joints, cough, shortness of breath, upper respiratory tract infection, low thyroid hormone levels (hypothyroidism), and dizziness.
Yervoy can cause the patient’s T cells to attack healthy cells throughout the body. Because of this, Yervoy can cause side effects that may involve any organ, can become serious or life-threatening, and may lead to death. Some of the serious side effects related to Yervoy include inflammation of the lungs, brain, liver (which can lead to liver failure), kidneys (which can lead to kidney failure), or colon (which can result in tears or holes in the intestine). Additionally, problems can arise with hormone glands (including thyroid, pituitary, and adrenal glands, as well as the pancreas), and nerves (which can lead to paralysis). Skin rash (which could become severe and life-threatening) and reactions related to the infusion may also occur. Patients should report any symptoms to their healthcare provider, who can then initiate actions to limit or reverse the side effects.
Patients may experience other side effects when Yervoy is used in combination with nivolumab (Opdivo)- including type 1 diabetes. For a more complete list of possible side effects, see the full prescribing information.
Additional information
Manufacturer
Bristol-Myers Squibb
Approval
FDA and EMA
Links to drug websites
Other references
- https://news.bms.com/press-release/corporatefinancial-news/us-food-and-drug-administration-approves-opdivo-nivolumab-ye-0
- https://news.bms.com/news/details/2020/Opdivo-nivolumab-Plus-Yervoy-ipilimumab-Demonstrates-Durable-Survival-Benefit-vs.-Chemotherapy-in-Patients-with-Previously-Untreated-Malignant-Pleural-Mesothelioma/default.aspx
- Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. Eggermont AMM, et al. The New England Journal of Medicine (2017)
- Nivolumab plus Ipilimumab versus Sunitini in Advanced Renal-Cell Carcinoma. Motzer RJ, et al. The New England Journal of Medicine (2018)
Last updated: March 13, 2024