How is this drug name pronounced?
Pembrolizumab: pem-broh-LIH-zoo-mab
Keytruda: kee-TROO-duh
What cancer(s) does this drug treat?
Keytruda has been approved for a number of cancer types and stages, in some cases as a single therapy, and in some cases in combination with another therapy.
Keytruda is approved for:
Melanoma
Non-small cell lung cancer
Head and neck squamous cell cancer
Classical Hodgkin lymphoma
Primary mediastinal B cell lymphoma
Bladder and urinary tract (urothelial cell) cancer
Microsatellite instability-high cancer
Stomach cancer
Esophagus cancer
Cervical cancer
Liver cancer
Biliary tract cancer
Merkel cell carcinoma
Kidney cancer
Endometrial carcinoma
Tumor mutational burden-high cancer
Cutaneous squamous cell carcinoma
Breast cancer
Advanced melanoma
Keytruda is approved for:
- Adult patients with advanced melanoma that is metastatic (cancer has spread from the original cancer site to other parts of the body) or cannot be completely removed by surgery.
- Adult and pediatric patients (12 years and older) with stage IIB, IIC, or III melanoma that was completely removed by surgery. In such cases, Keytruda is used to help keep melanoma from coming back.
Advanced non-small cell lung cancer
Keytruda is approved for:
- Patients with nonsquamous non-small cell lung cancer that has spread and does not have an abnormal EGFR or ALK gene. In such cases, Keytruda may be used in combination with the chemotherapy drug pemetrexed (Alimta) and a platinum chemotherapy as a first treatment.
- Patients with squamous non-small cell lung cancer that has spread. In such cases, Keytruda may be used in combination with the chemotherapies carboplatin (Paraplatin) and paclitaxel (Taxol or Onxal) as a first treatment.
-
Patients with non-small cell lung cancer that:
- tests positive for the PD-L1 molecule, AND
- does not have an abnormal EGFR or ALK gene, AND
- either has not spread outside the chest (stage III) but cannot be treated with surgery or chemotherapy with radiation, or has spread outside the chest.
In such cases, Keytruda may be used alone as a first treatment.
- Patients with non-small cell lung cancer that tests positive for the PD-L1 molecule and has spread. The patient must have already tried chemotherapy containing platinum and it either did not work or stopped working. If the patient’s tumor has an abnormal EGFR or ALK gene, the patient has to have also tried an FDA-approved therapy for tumors with such abnormal genes prior to receiving Keytruda.
- Patients with non-small cell lung cancer tumors that can be removed by surgery (resectable). In such cases, Keytruda is used in combination with a platinum-containing chemotherapy prior to surgery (neoadjuvant treatment) and then is continued alone after surgery to prevent the cancer from returning (adjuvant treatment).
- Patients with stage IB, II, or IIIA non-small cell lung cancer. Keytruda is used by itself after surgical removal of disease and platinum-based chemotherapy.
Head and neck squamous cell cancer
Keytruda is approved for:
- Patients with head and neck squamous cell cancer that has spread or come back, cannot be removed by surgery, and tests positive for the PD-L1 molecule. In such cases, Keytruda may be used alone as a first treatment.
- Patients with head and neck squamous cell cancer whose cancer has spread or come back after treatment with chemotherapy that contains platinum.
- Patients with head and neck squamous cell cancer that has spread or come back and cannot be removed by surgery. In such cases, Keytruda may be used in combination with the chemotherapy drug fluorouracil (Adrucil) and a chemotherapy containing platinum as a first treatment.
Classical Hodgkin lymphoma
Keytruda is approved for:
- Adult patients with classical Hodgkin lymphoma that either has not responded to treatment, or got better, but then came back.
- Pediatric patients with classical Hodgkin lymphoma that either has not responded to treatment, or got better, but then came back after two or more types of treatment.
Primary mediastinal B cell lymphoma
Keytruda is approved for:
- Adult and pediatric patients with primary mediastinal B cell lymphoma that either has not responded to treatment, or has gotten better but then came back after two or more types of treatment. Keytruda is NOT recommended for patients with primary mediastinal B cell lymphoma who require urgent cytoreductive therapy to control the blood cell count.
Bladder and urinary tract (urothelial cell) cancer
Keytruda is approved for:
-
Patients with advanced urothelial carcinoma (the most common type of bladder and urinary tract cancer) that has grown or spread and cannot be removed by surgery:
- who have been treated with chemotherapy containing platinum, and it did not work or stopped working, OR
- who cannot be treated with any chemotherapy containing platinum.
- Patients with advanced urothelial carcinoma (the most common type of bladder and urinary tract cancer) that has grown or spread. In such cases, Keytruda is used in combination with enfortumab vedotin (Padcev).
-
Patients with bladder cancer that has not yet spread to nearby tissue (called carcinoma in situ), but is at a high risk for spreading (high-risk, non-muscle invasive bladder cancer):
- who have tried Bacillus Calmette-Guerin treatment, and it either did not work or stopped working, AND
- who either cannot or choose not to have their bladder surgically removed.
Microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) cancer, including colorectal cancer and endometrial cancer
Keytruda is approved for:
- Adult and pediatric patients with previously treated microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) solid tumors (including colorectal, endometrial, biliary, gastric or gastroesophageal junction, pancreatic, small intestinal, prostate, breast, bladder, renal cell, small cell lung, sarcoma, mesothelioma, thyroid, brain, ovarian, cervical, neuroendocrine, and other cancers), whose cancer has spread or cannot be removed by surgery and who have no other satisfactory treatment options available.
- Patients with previously untreated microsatellite instability-high (MSI-H) or mismatch repair- deficient (dMMR) colon or rectal cancer that cannot be removed by surgery or has spread to other parts of the body. In such cases, Keytruda may be used as a first treatment.
- Patients with previously treated, advanced, microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) endometrial cancer that cannot be removed by surgery or treated with radiation. In such cases, Keytruda may be used by itself.
Advanced stomach cancer
Keytruda is approved for:
- Patients with a type of stomach cancer called gastric or gastroesophageal junction adenocarcinoma that has grown or spread and cannot be removed by surgery, and that tests positive for the HER2 molecule and the PD-L1 molecule. In such cases, Keytruda is used in combination with trastuzumab (e.g., Herceptin) and chemotherapy containing platinum and fluoropyrimidine.
- Patients with gastric or gastroesophageal junction adenocarcinoma that has grown or spread and cannot be removed by surgery, and that tests negative for the HER2 molecule. In such cases, Keytruda is used in combination with chemotherapy containing platinum and fluoropyrimidine.
Advanced esophagus cancer
Keytruda is approved for:
- Patients with esophageal cancer that has spread to nearby lymph nodes or other parts of the body, cannot be removed by surgery or treated with concurrent chemotherapy and radiation, and tests positive for the PD-L1 molecule, and who have received at least one treatment that either did not work or stopped working. In such cases, Keytruda is used on its own.
- Patients with esophageal cancer that has spread to nearby lymph nodes or other parts of the body and cannot be removed by surgery or treated with concurrent chemotherapy and radiation. In such cases, Keytruda may be used in combination with chemotherapy containing platinum and fluoropyrimidine.
Advanced cervical cancer
Keytruda is approved for:
- Patients with FIGO 2014 Stage III-IVA cervical cancer. In such cases, Keytruda is used in combination with chemotherapy and radiotherapy.
- Patients with cervical cancer that has come back or spread or cannot be removed by surgery and tests positive for the PD-L1 molecule, and who have received chemotherapy that either did not work or stopped working. In such cases, Keytruda is used on its own.
- Patients with cervical cancer that has come back or spread to other parts of the body and tests positive for the PD-L1 molecule. In such cases, Keytruda is used in combination with chemotherapy and, in some cases, bevacizumab (Avastin, Mvasi, Zirabev).
Advanced liver cancer
Keytruda is approved for:
- Patients with hepatocellular carcinoma (liver cancer) caused by a hepatitis B infection who have been treated before, but not with anti-PD-1/PD-L1 therapy.
Biliary tract cancer
Keytruda is approved for:
- Patients with biliary tract cancer that is locally advanced and cannot be surgically removed (unresectable), or that has spread to other parts of the body (metastatic). In such cases, Keytruda is used in combination with gemcitabine and cisplatin chemotherapy.
Advanced Merkel cell carcinoma
Keytruda is approved for:
- Adult and pediatric patients with Merkel cell carcinoma (a type of skin cancer) that has come back or spread.
Advanced kidney cancer
Keytruda is approved for:
- Patients with renal cell carcinoma (kidney cancer) that has spread or cannot be removed by surgery. In such cases, Keytruda may be used in combination with axitinib (Inlynta) as a first treatment.
- Patients with renal cell carcinoma (kidney cancer) whose cancer-affected kidney and other tissue the cancer had spread to (metastases) had been partially or completely removed by surgery, and who are at intermediate or high risk for the cancer to come back. In such cases, patients may be treated with Keytruda following surgery.
Advanced endometrial carcinoma
Keytruda is approved for:
- Patients with advanced endometrial cancer or endometrial cancer that has come back. In such cases, Keytruda may be used in combination with carboplatin and paclitaxel chemotherapy, followed by just Keytruda.
- Patients with advanced endometrial cancer that is not microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR), and who have received prior treatment that did not work or stopped working, and who cannot receive surgery or radiation. In such cases, Keytruda may be used in combination with lenvatinib (Lenvima).
- Patients with previously treated advanced endometrial cancer that is microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR), and whose cancer cannot be removed by surgery or treated with radiation. In such cases, Keytruda may be used by itself.
Tumor mutational burden-high cancer
Keytruda is approved for:
-
Adult and pediatric patients with solid tumors whose cancer:
- cannot be removed by surgery or has spread to other parts of the body, AND
- has a high number of genetic mutations (changes) in the cancer cells, AND
- who have received prior treatment, but it did not work or stopped working, and no other satisfactory treatment options are available.
Cutaneous squamous cell carcinoma
Keytruda is approved for:
- Patients with cutaneous squamous cell carcinoma (a type of skin cancer) that has spread or come back, and could not be removed by surgery or be cured by radiation.
Triple-negative breast cancer
Keytruda is approved for:
- Patients with triple-negative breast cancer (cancer that tests negative for estrogen hormone receptor, progesterone hormone receptor, and human epidermal growth factor receptor 2 [HER2]) that has come back and grown at the original tumor site or spread to other parts of the body, cannot be removed by surgery, and tests positive for the PD-L1 molecule. In such cases, Keytruda is used in combination with chemotherapy.
- Patients with early-stage triple-negative breast cancer who are at a high risk for the cancer to become worse. In such cases, Keytruda is used in combination with chemotherapy before surgical removal of all known disease and by itself after surgery.
Limitations of Use
Age: The safety and efficacy of Keytruda has been established in pediatric patients with classical Hodgkin lymphoma, primary mediastinal B cell lymphoma, Merkel cell carcinoma, microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) cancers, and tumor mutational burden high (TMB-H) cancers. The safety and efficacy of Keytruda in pediatric patients with MSI-H and TMB-H central nervous system cancers have not been established. The safety and efficacy of Keytruda in pediatric patients with any other cancer type approved for treatment with Keytruda have not been established.
Pregnancy/Breastfeeding: Keytruda can cause harm to a fetus, and is not recommended for use during pregnancy. The risks associated with Keytruda during breastfeeding are not known and cannot be ruled out; due to the potential for serious adverse reactions in the breastfed child, women are advised not to breastfeed during treatment and for 4 months after the last dose of Keytruda.
Complications of stem cell transplant: Serious and life-threatening complications, which can lead to death, can occur in patients who have received a stem cell transplant from a stem cell donor before or after being treated with Keytruda.
Solid organ transplant rejection: Treatment with Keytruda can increase the risk of rejection in patients who have received a solid organ transplant or other transplant (including corneal graft).
What type of immunotherapy is this?
- PD-1 blockade
How does this drug work?
- Target: PD-1
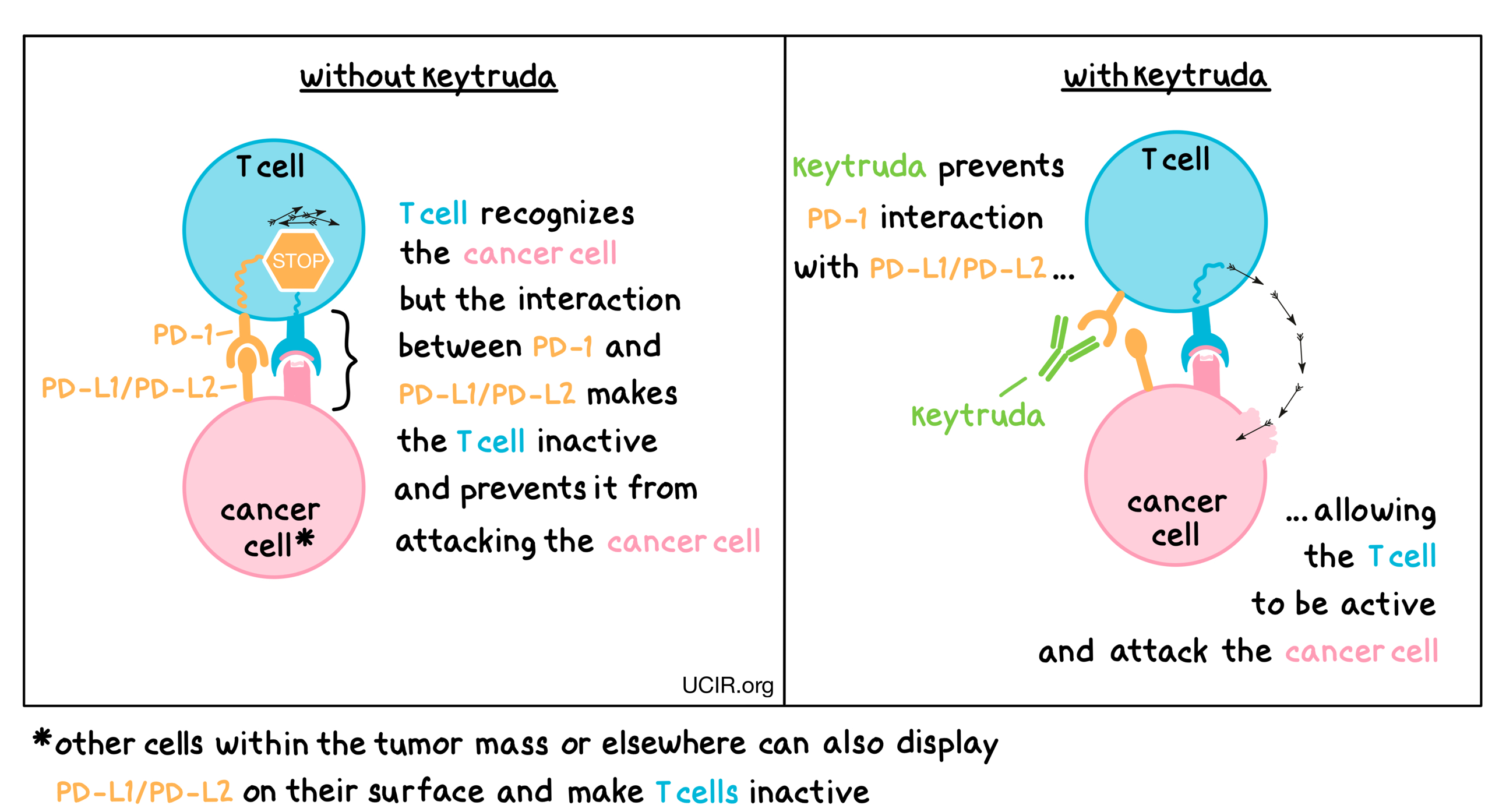
Keytruda is an antibody that attaches to a molecule called PD-1, which is present on the surface of T cells – the primary immune cells involved in killing cancer cells. In healthy tissues, PD-1 acts as a brake that keeps T cells from creating an immune reaction that gets out of control. However, cancers can hijack this safety mechanism and prevent T cells from doing their job – killing the cancer cells. This can happen when cancer cells or other cells within the tumor mass display molecules called PD-L1 or PD-L2 on their surface. When PD-L1 or PD-L2 interact with PD-1 on T cells, the T cells become inactive and do not attack the cancer cells. Keytruda binds to the PD-1 molecules on T cells in such a way that it prevents the interaction between PD-1 and PD-L1/PD-L2 and allows the T cells to be active and attack the cancer cells.
How is this drug given to the patient?
Keytruda is administered via a tube into a vein (intravenous infusion, or I.V.) over 30 minutes every three weeks or every six weeks, depending on the cancer type, treatment combination, dose, and the age of the patient (adult or pediatric).
What are the observed clinical results?
For:
Advanced melanoma (metastatic or not removable by surgery)
Advanced melanoma (completely removed by surgery)
Advanced non-small cell lung cancer (squamous and non-squamous)
Head and neck squamous cell cancer
Classical Hodgkin lymphoma
Primary mediastinal B cell lymphoma
Bladder and urinary tract (urothelial cell) cancer
Microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) cancer, including colorectal cancer
Advanced stomach cancer
Advanced esophagus cancer
Advanced cervical cancer
Advanced liver cancer
Biliary tract cancer
Advanced Merkel cell carcinoma
Advanced kidney cancer
Advanced endometrial carcinoma
Tumor mutational burden-high (TMB-H) cancer
Cutaneous squamous cell carcinoma
Triple-negative breast cancer
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed. The long-term clinical trial data currently available is limited, and more clinical trials are ongoing.
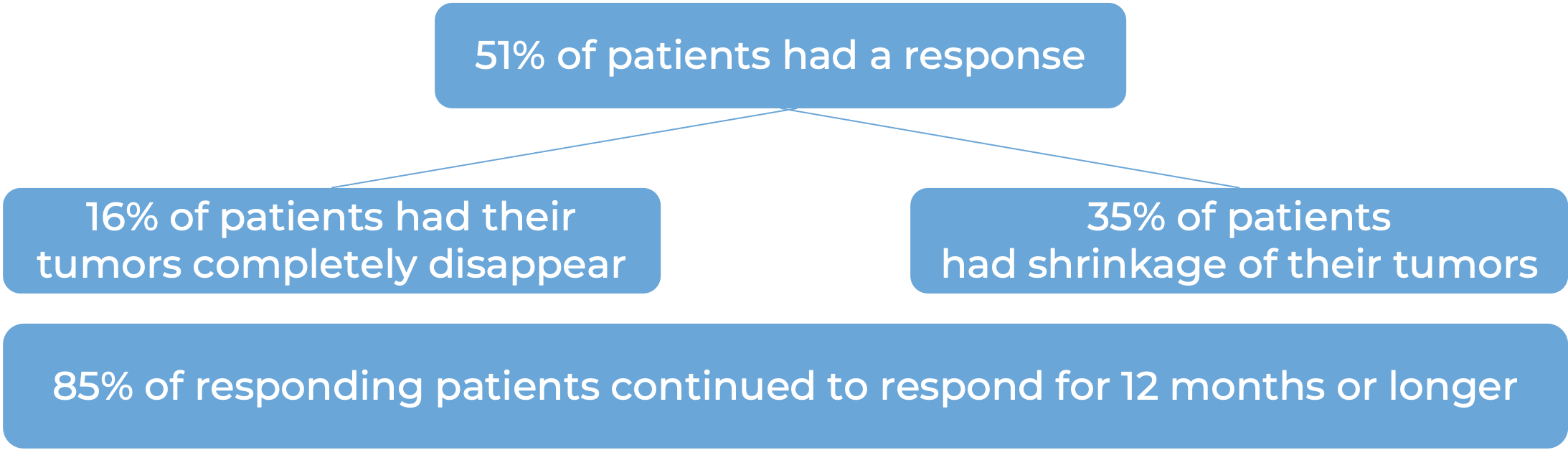
Advanced melanoma (metastatic or not removable by surgery)
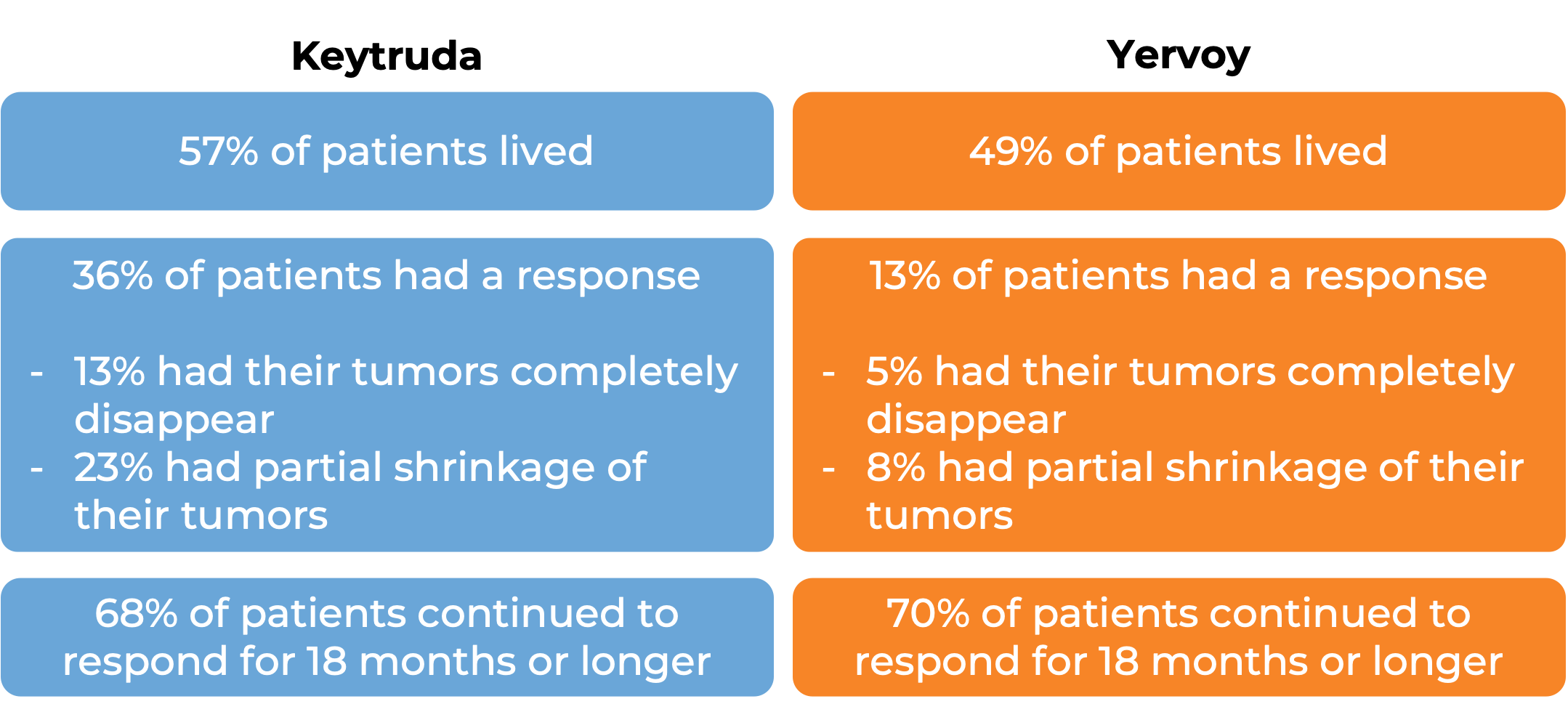
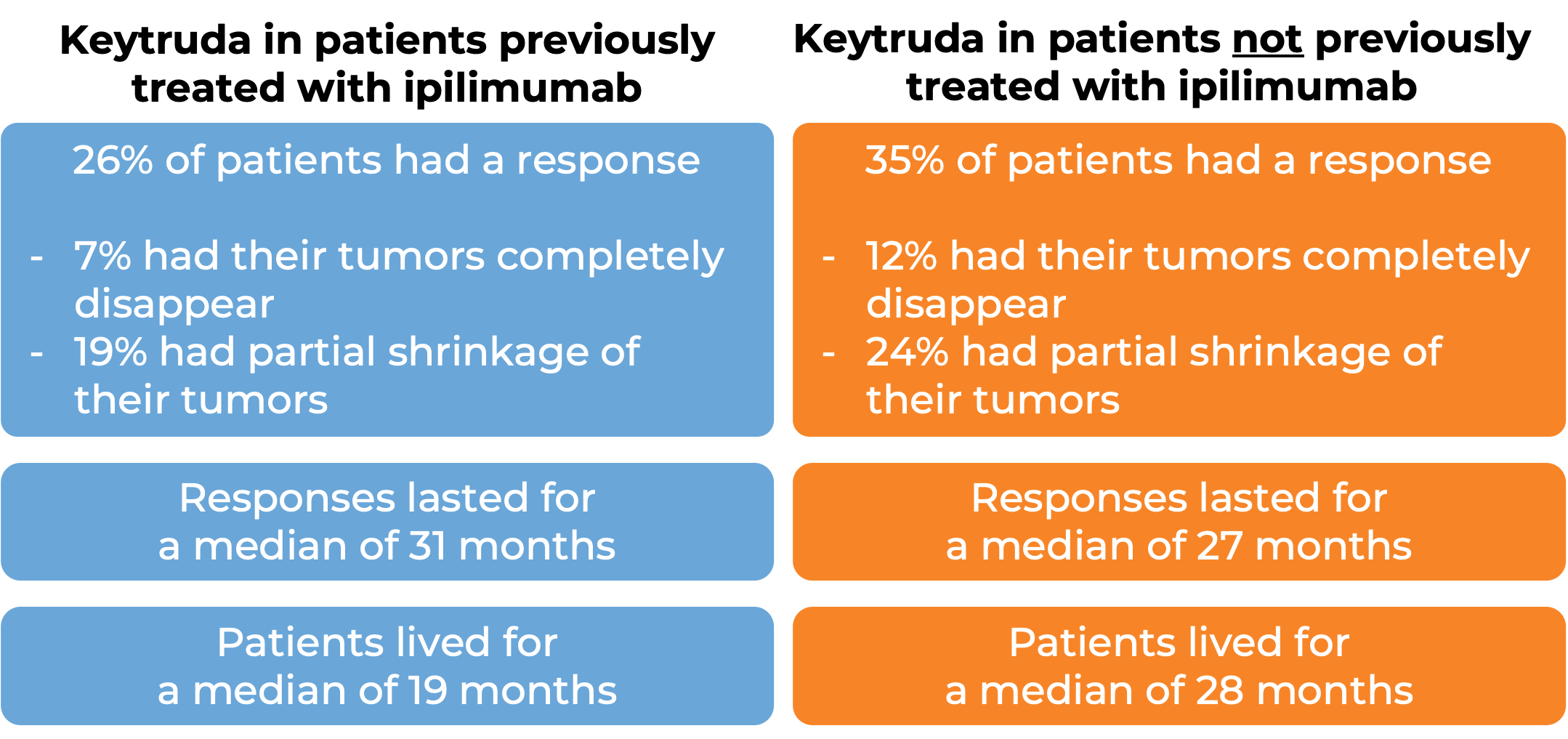
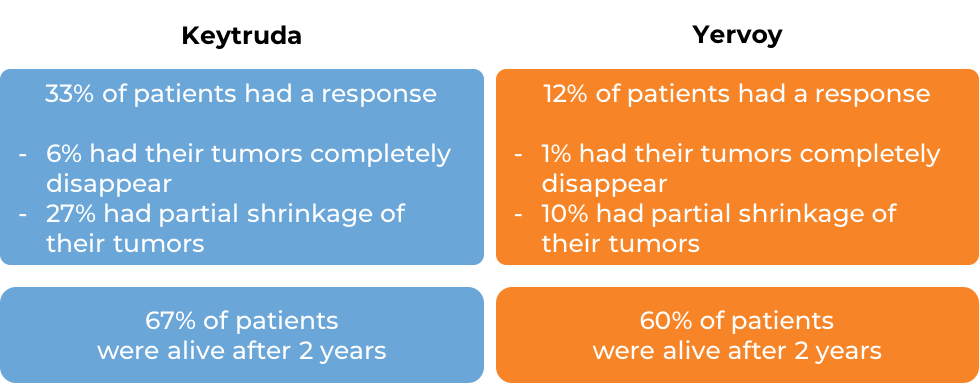
In a clinical trial, 834 patients with melanoma that could not be removed by surgery or had spread to other parts of the body, and who had no prior treatment with Yervoy (ipilimumab) – another form of immunotherapy) received either Keytruda or Yervoy. At a median follow-up of 23 months:
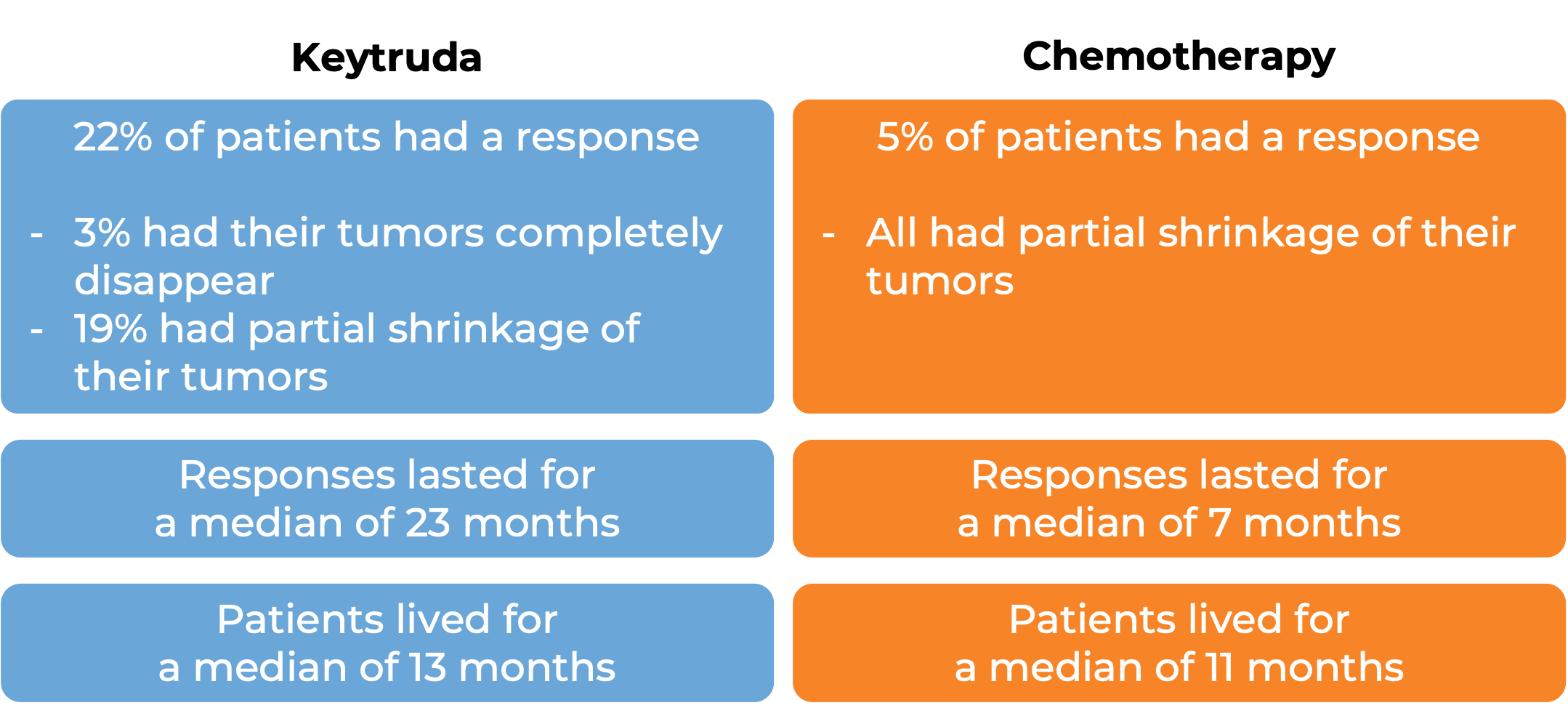
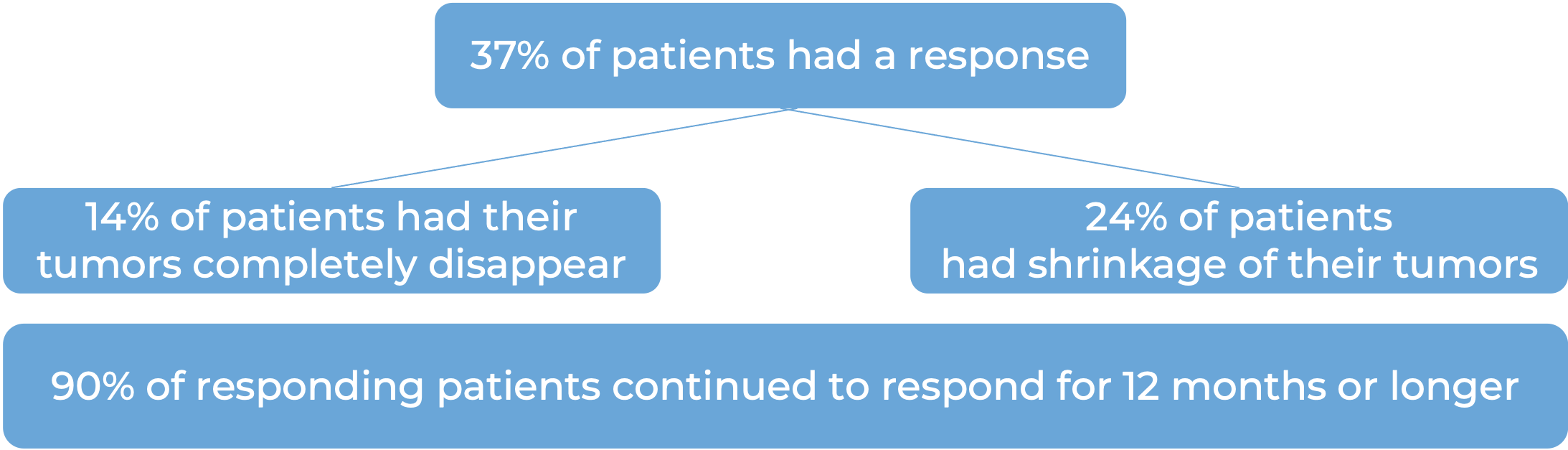
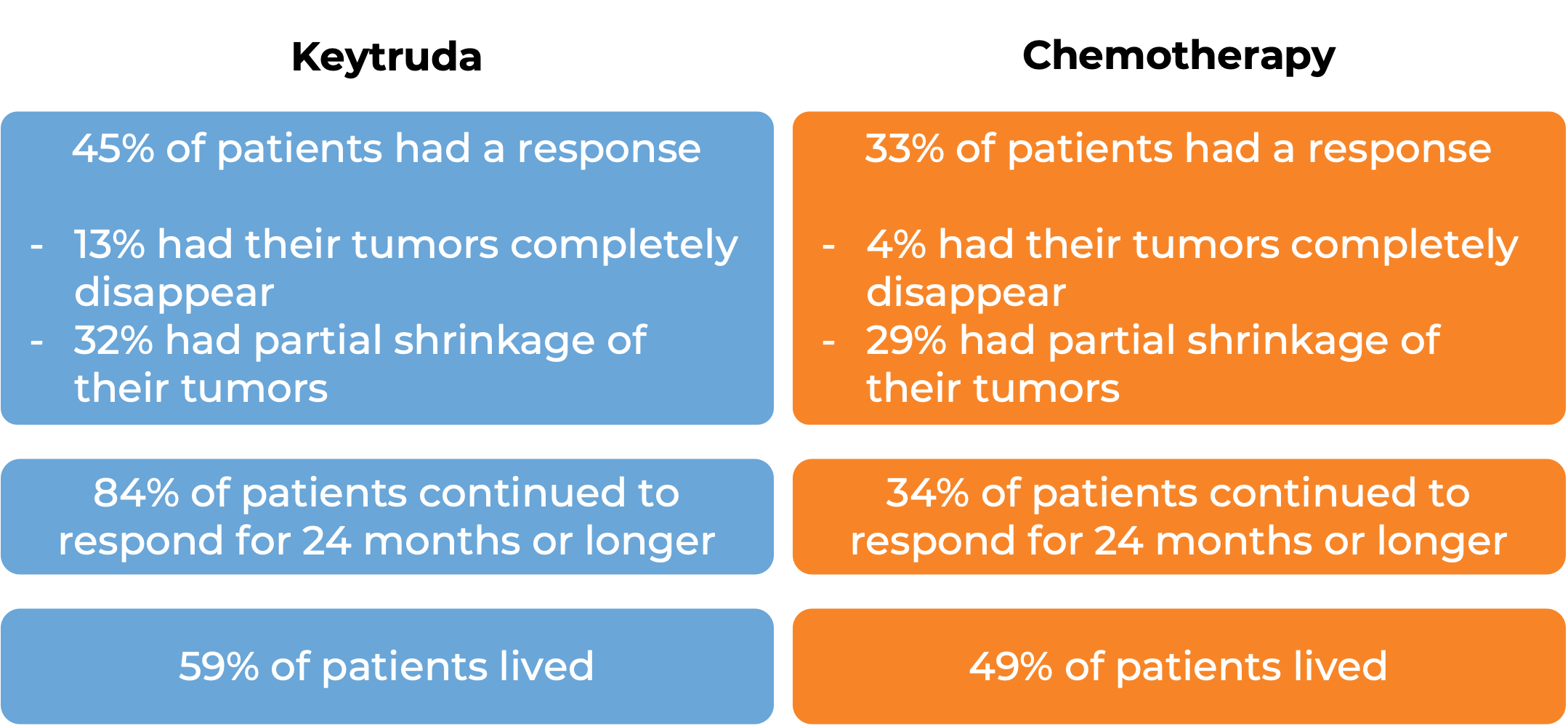
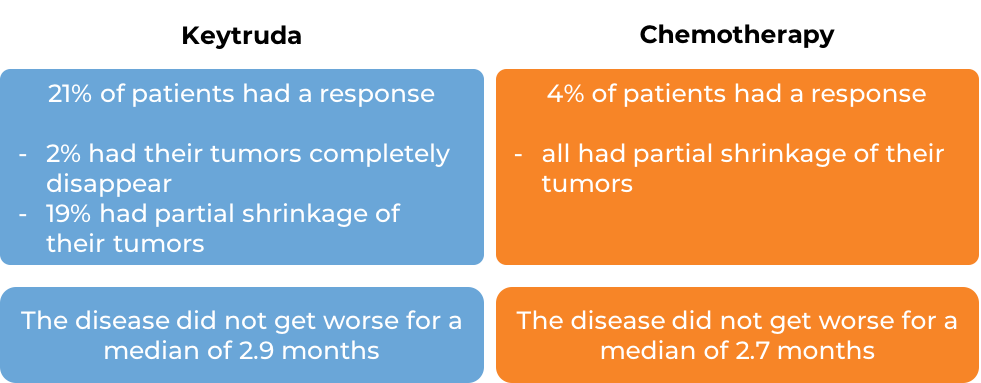
In another clinical trial, 540 patients with melanoma that could not be removed by surgery or had spread to other parts of the body, and did not respond to or progressed on Yervoy (ipilimumab) received either Keytruda or chemotherapy. At a median follow-up of 18 months:
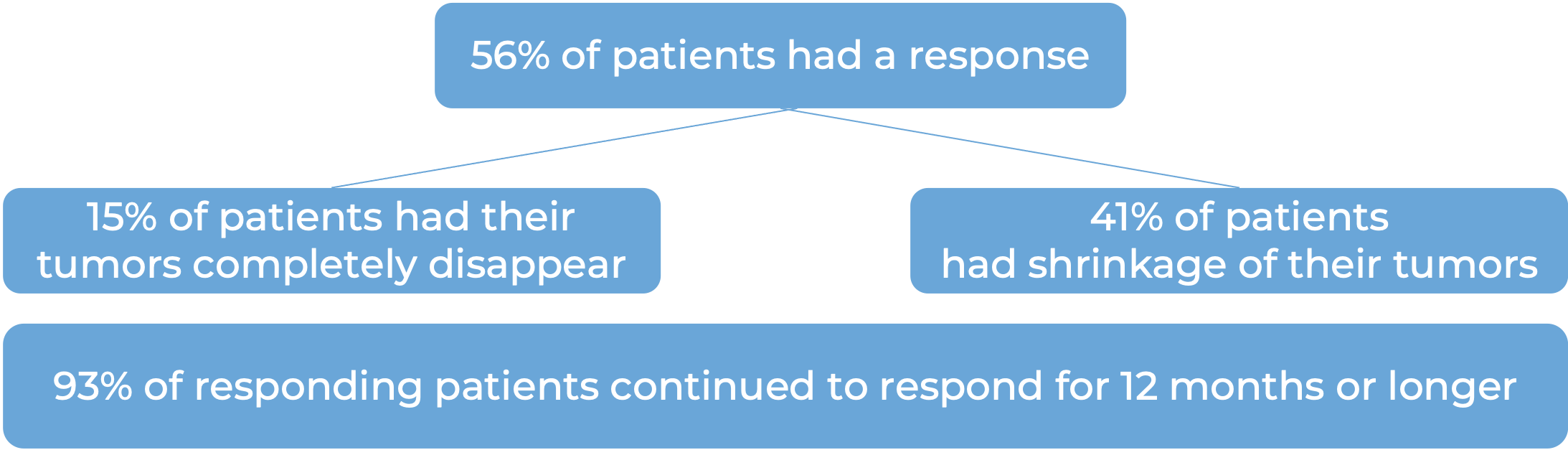
Advanced melanoma (completely removed by surgery)
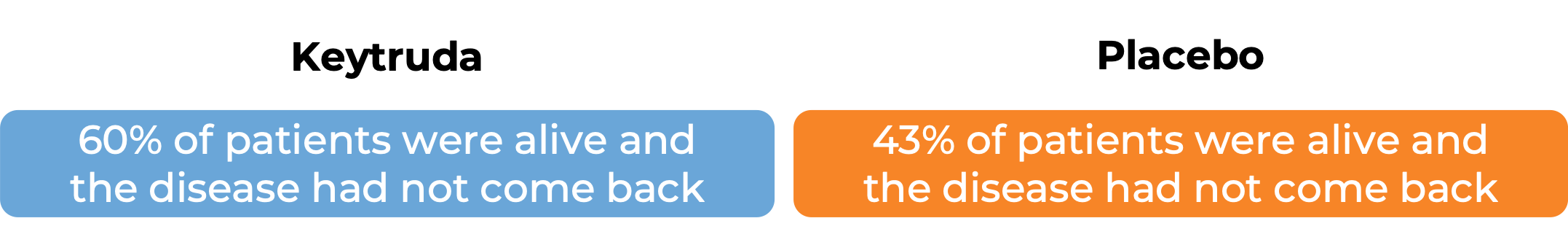
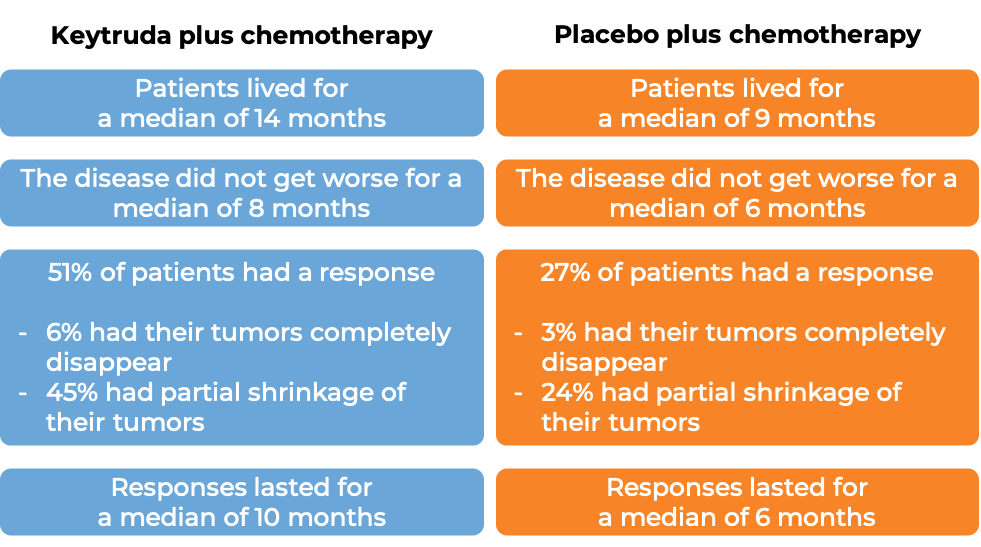
In a clinical trial, 976 adult and pediatric patients (12 years and older) with previously untreated stage IIB or IIC melanoma (deep or ulcerated tumors at original site) that was completely removed by surgery, were treated with or placebo for up to one year. At a median follow-up of 14 months:
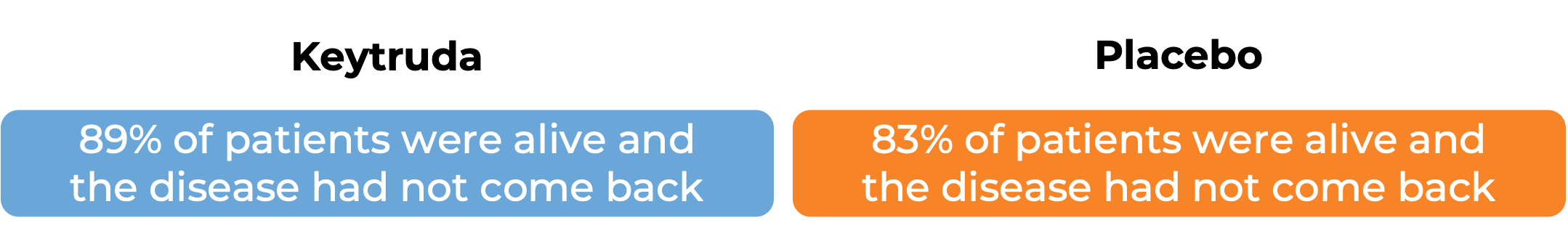
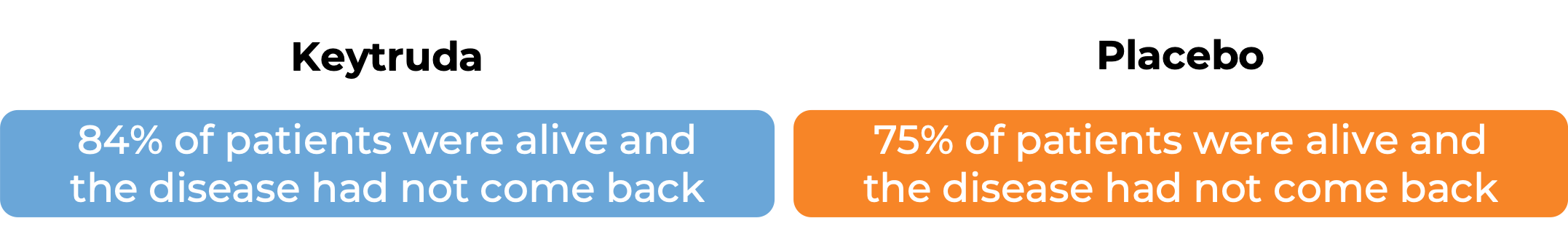
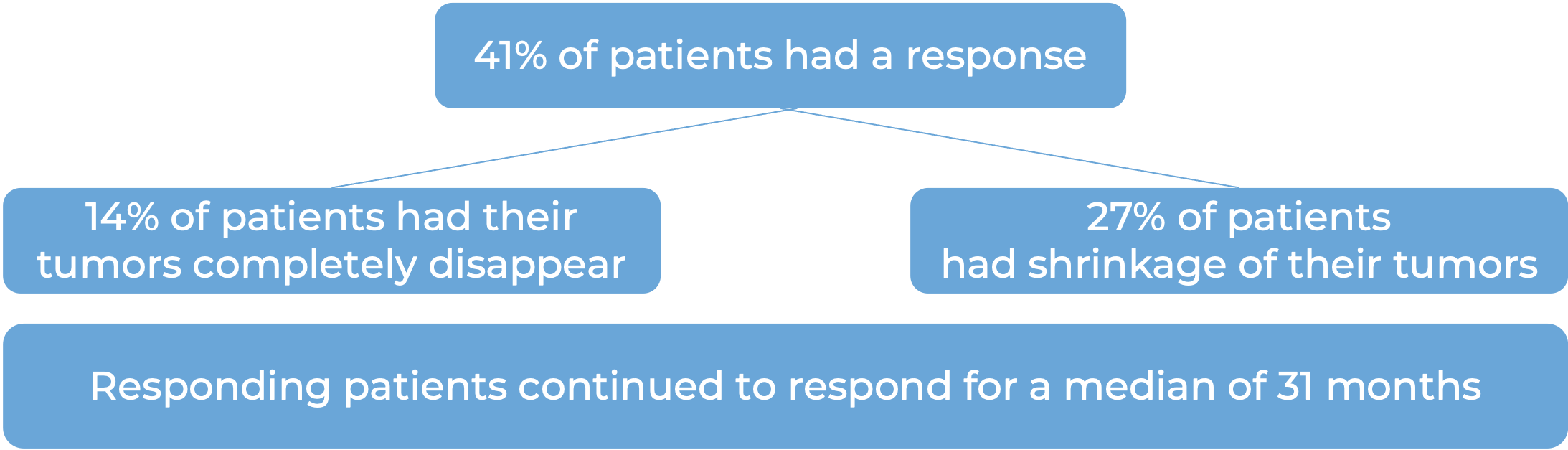
In another clinical trial, 1019 patients with stage III melanoma that was, together with the lymph nodes that contained cancer, completely removed by surgery, were treated with Keytruda or placebo. At a median follow-up of 15 months:
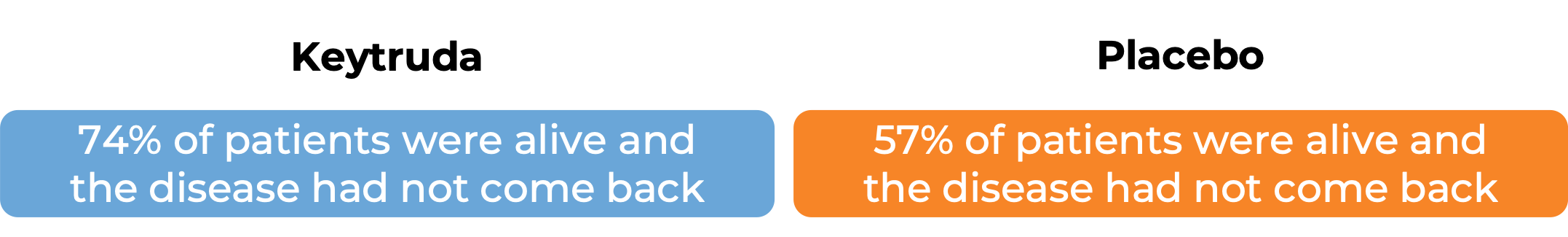
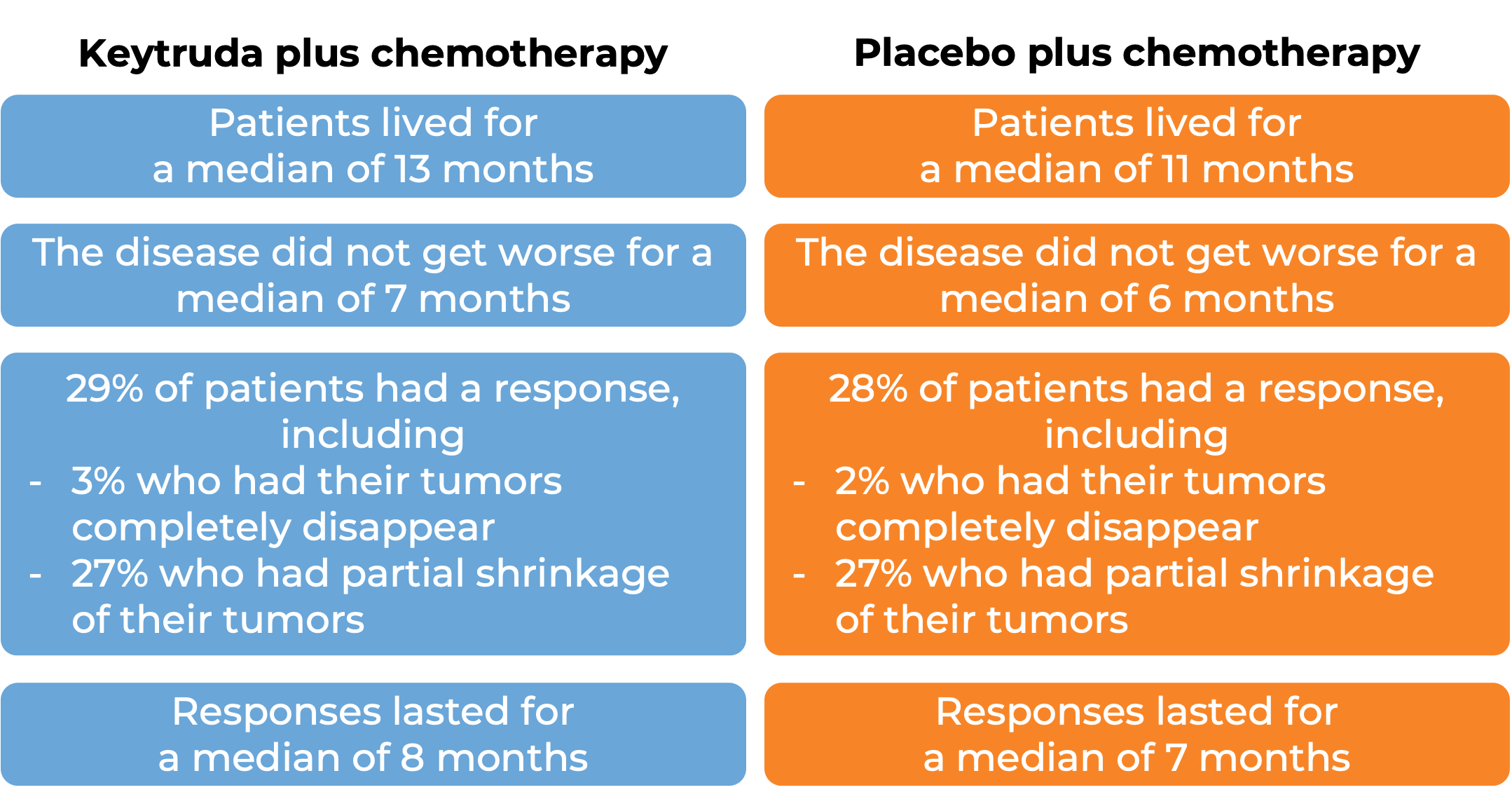
Advanced non-small cell lung cancer (squamous and non-squamous)
Keytruda with chemotherapy
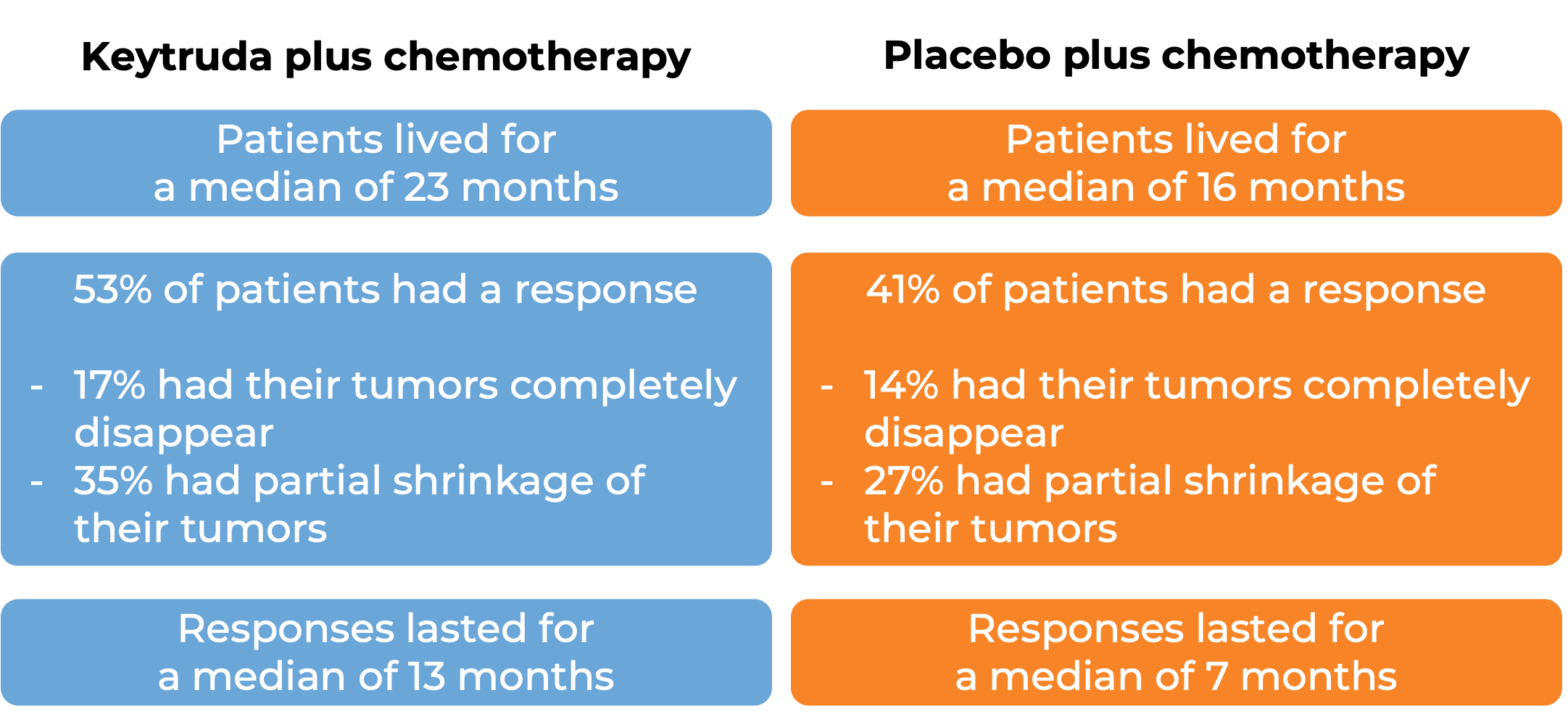
In a clinical trial, 616 patients with metastatic non-squamous non-small cell lung cancer who had not received treatment for metastatic disease were treated with
- Keytruda plus chemotherapy (pemetrexed and cisplatin or carboplatin), OR
- placebo plus chemotherapy.
At a median follow-up of 11 months:
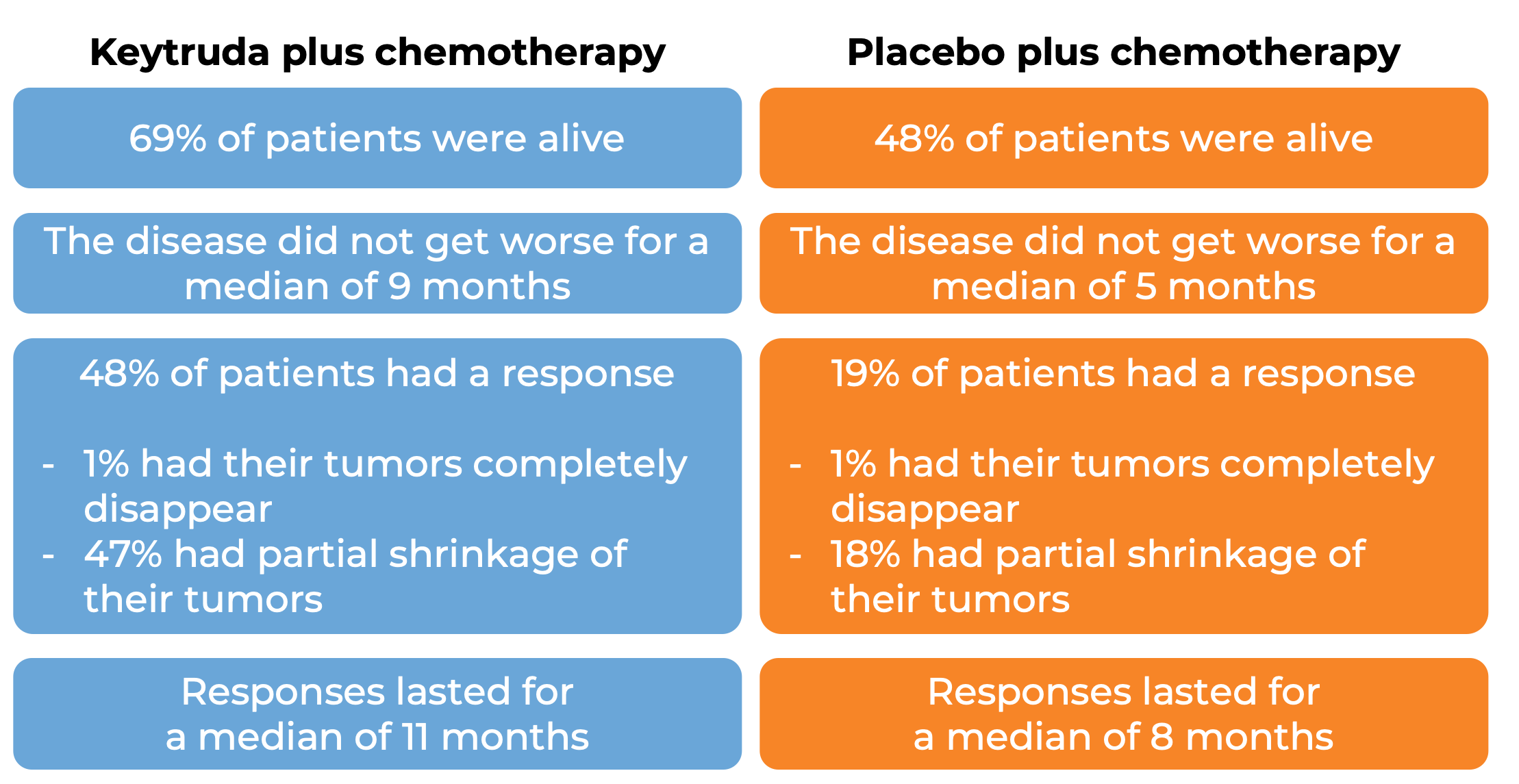
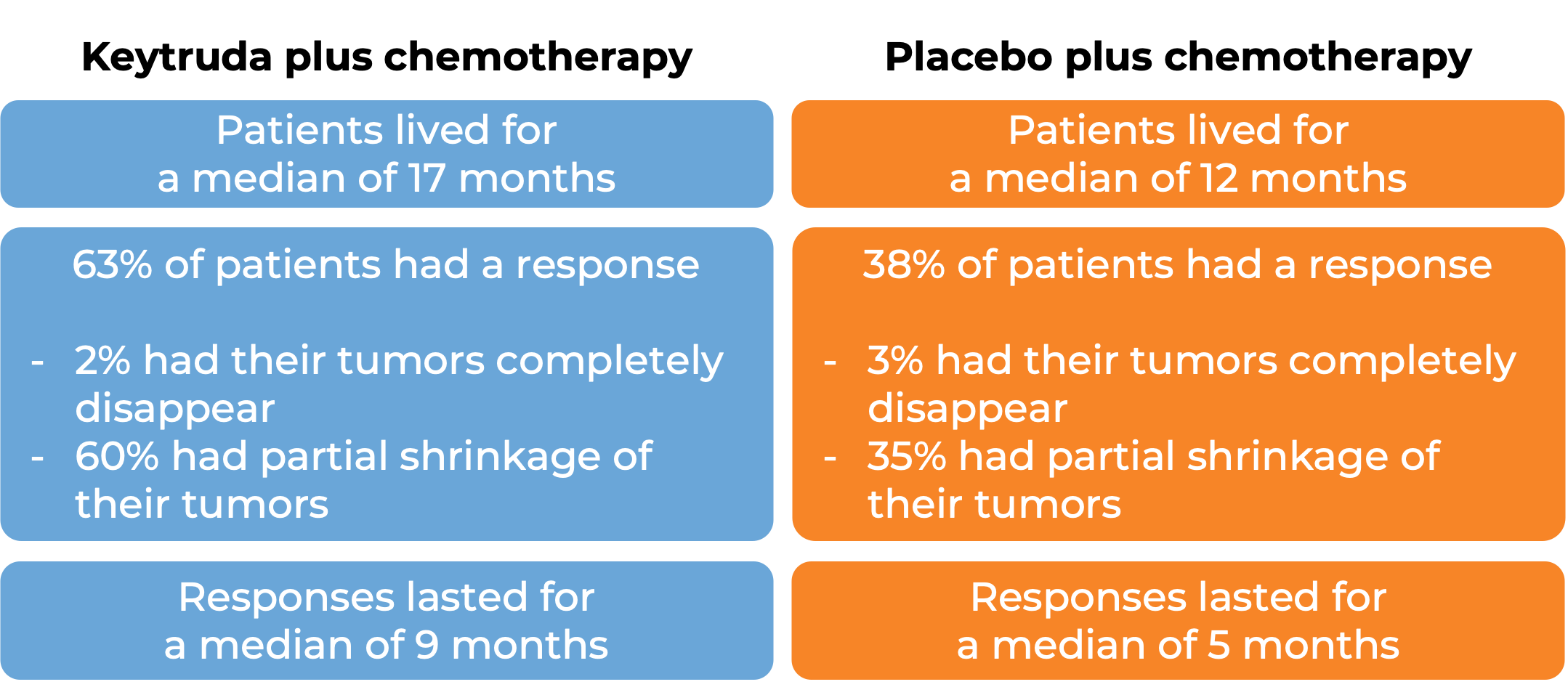
In another clinical trial, 559 patients with metastatic squamous non-small cell lung cancer who had not received treatment for metastatic disease were treated with
- Keytruda plus chemotherapy (carboplatin and paclitaxel or paclitaxel protein-bound), OR
- placebo plus chemotherapy. At a median follow-up of 8 months:
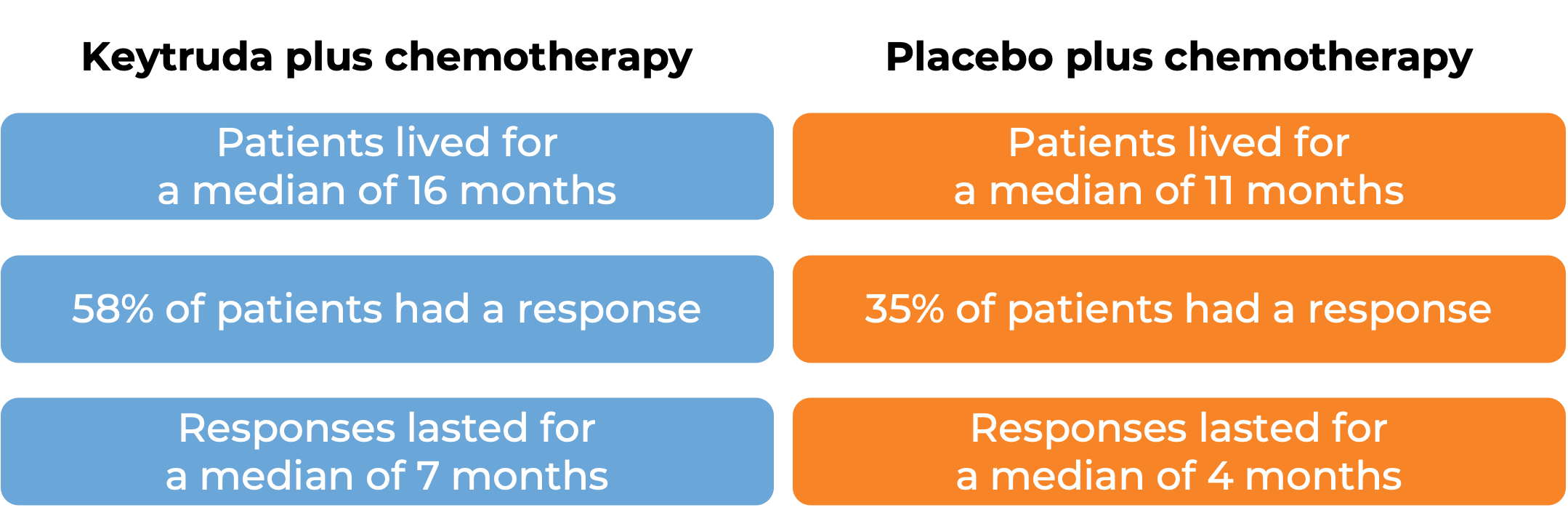
Keytruda alone as a first treatment
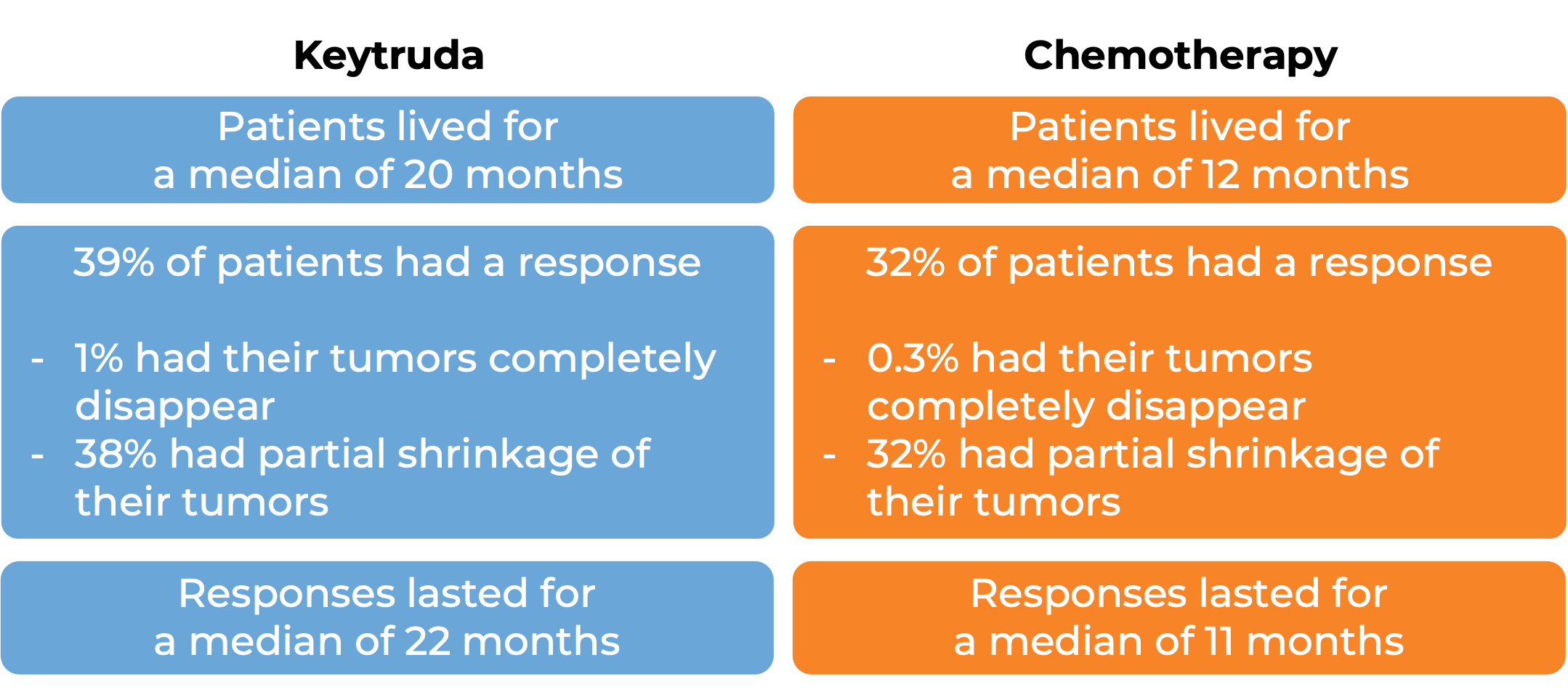
In a clinical trial, 1274 patients with non-small cell lung cancer that tested positive for PD-L1 and that was either stage III and could not be treated with surgery or chemoradiation or was metastatic were treated with Keytruda or chemotherapy (pemetrexed and carboplatin followed by optional pemetrexed OR paclitaxel and carboplatin followed by optional pemetrexed). In that trial:
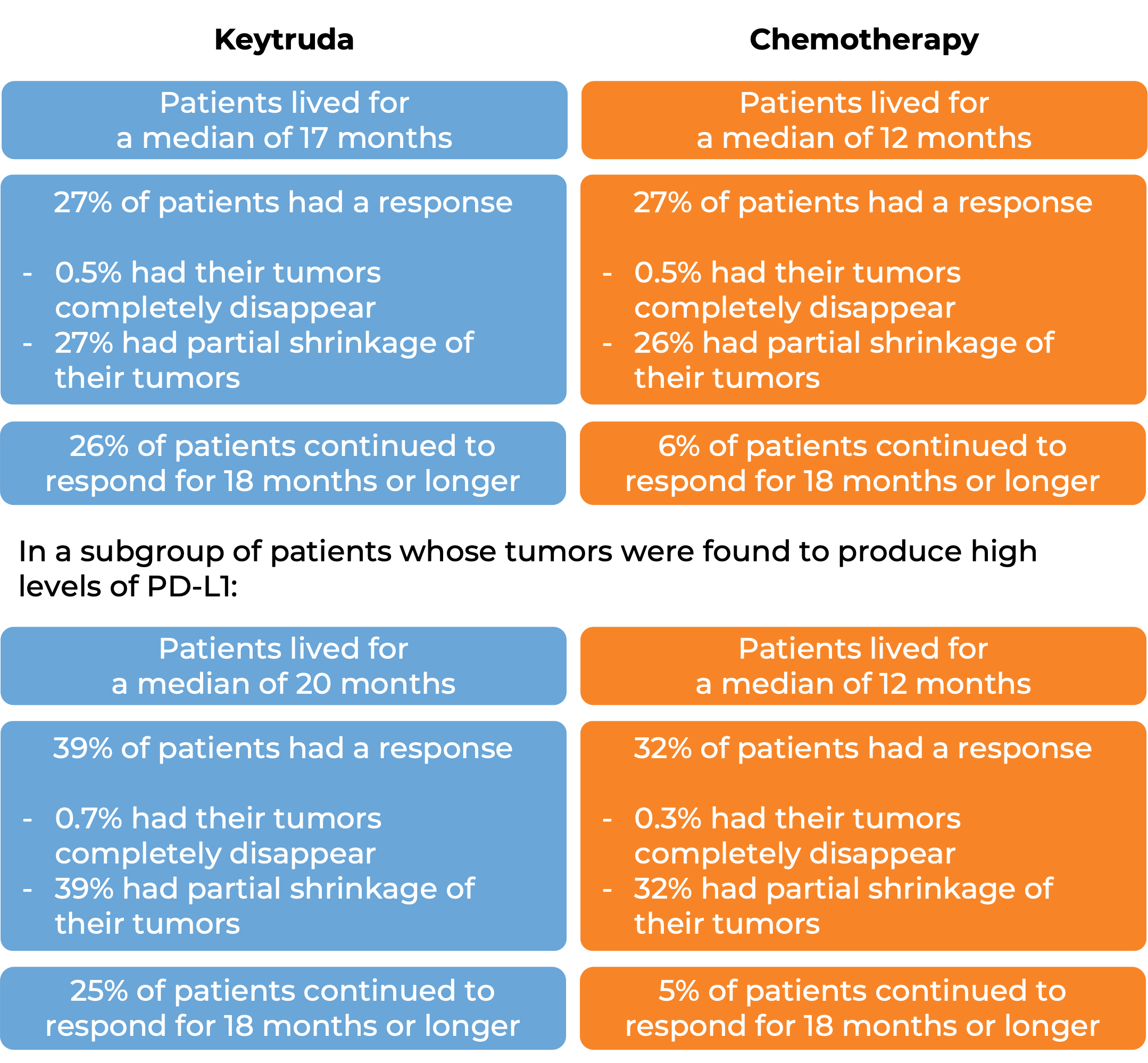
In another clinical trial, 305 patients with metastatic non-small cell lung cancer that had high levels of PD-L1 and that was previously untreated were treated with Keytruda or a platinum-based chemotherapy. At a median follow-up of 25 months:
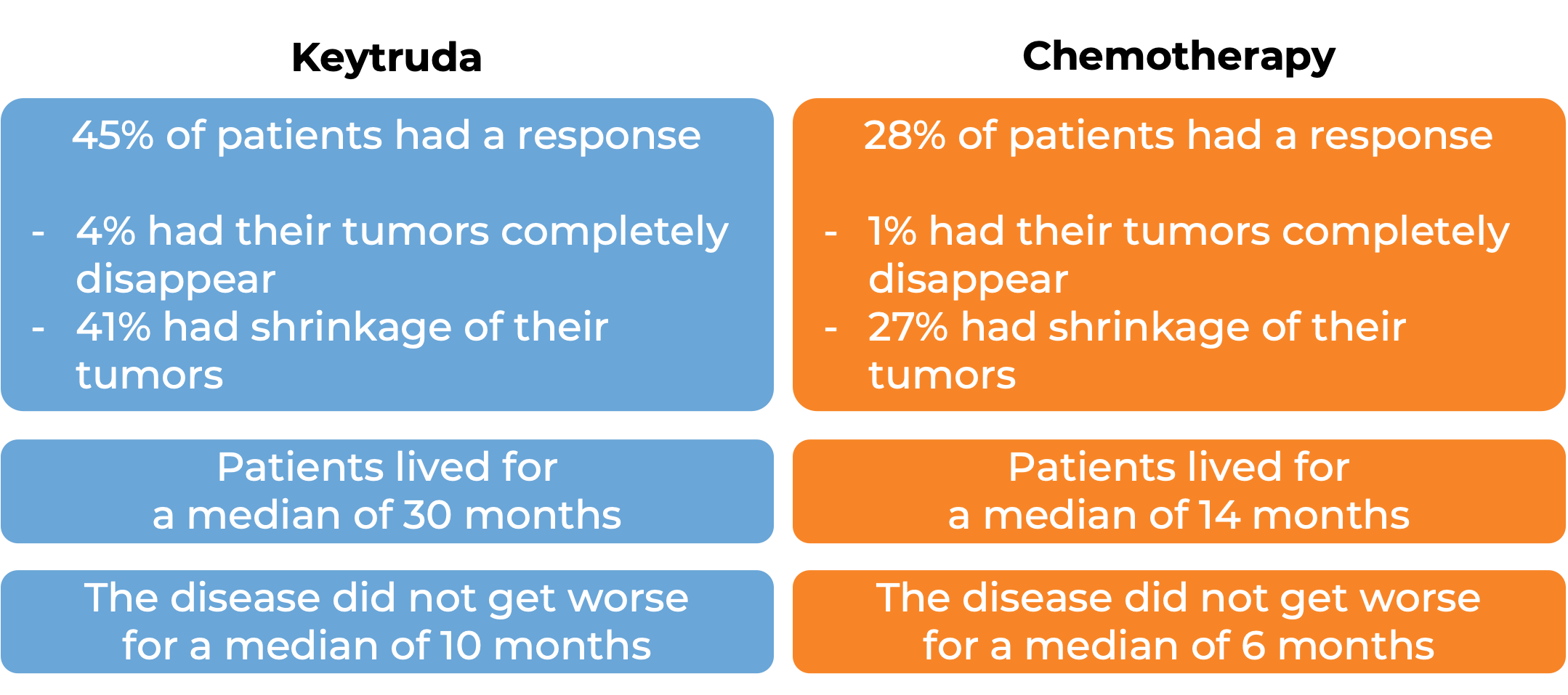
Keytruda for previously treated non-small cell lung cancer
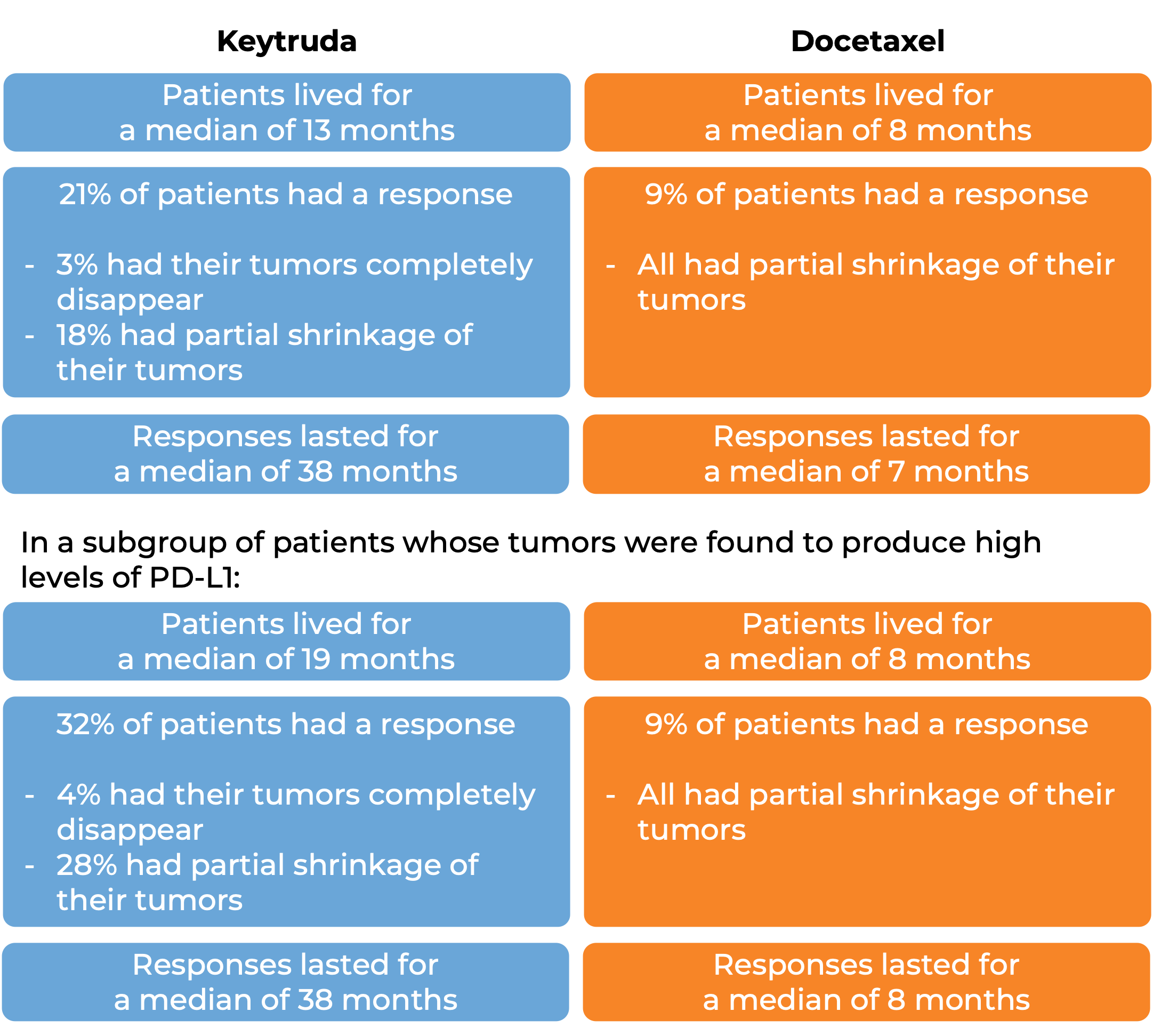
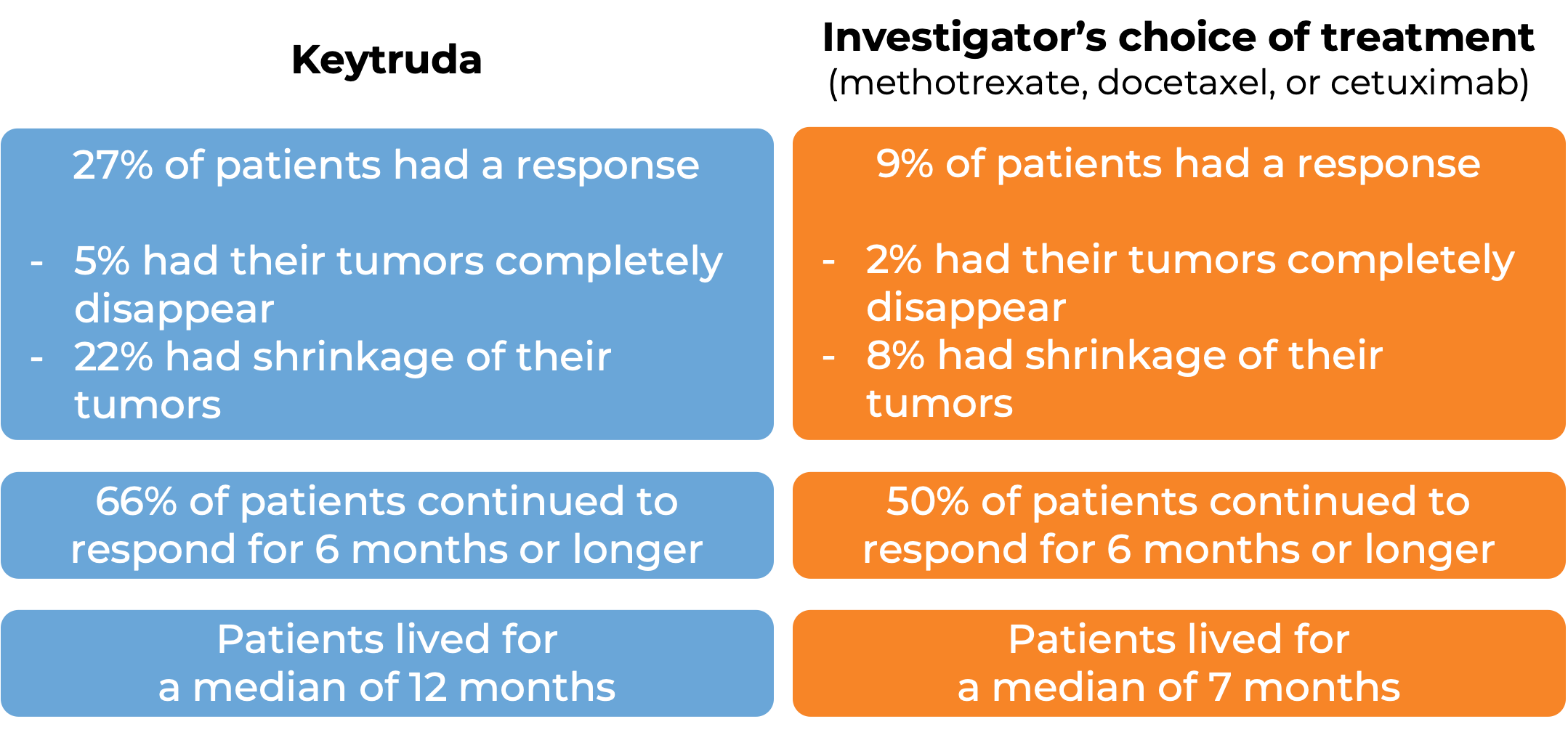
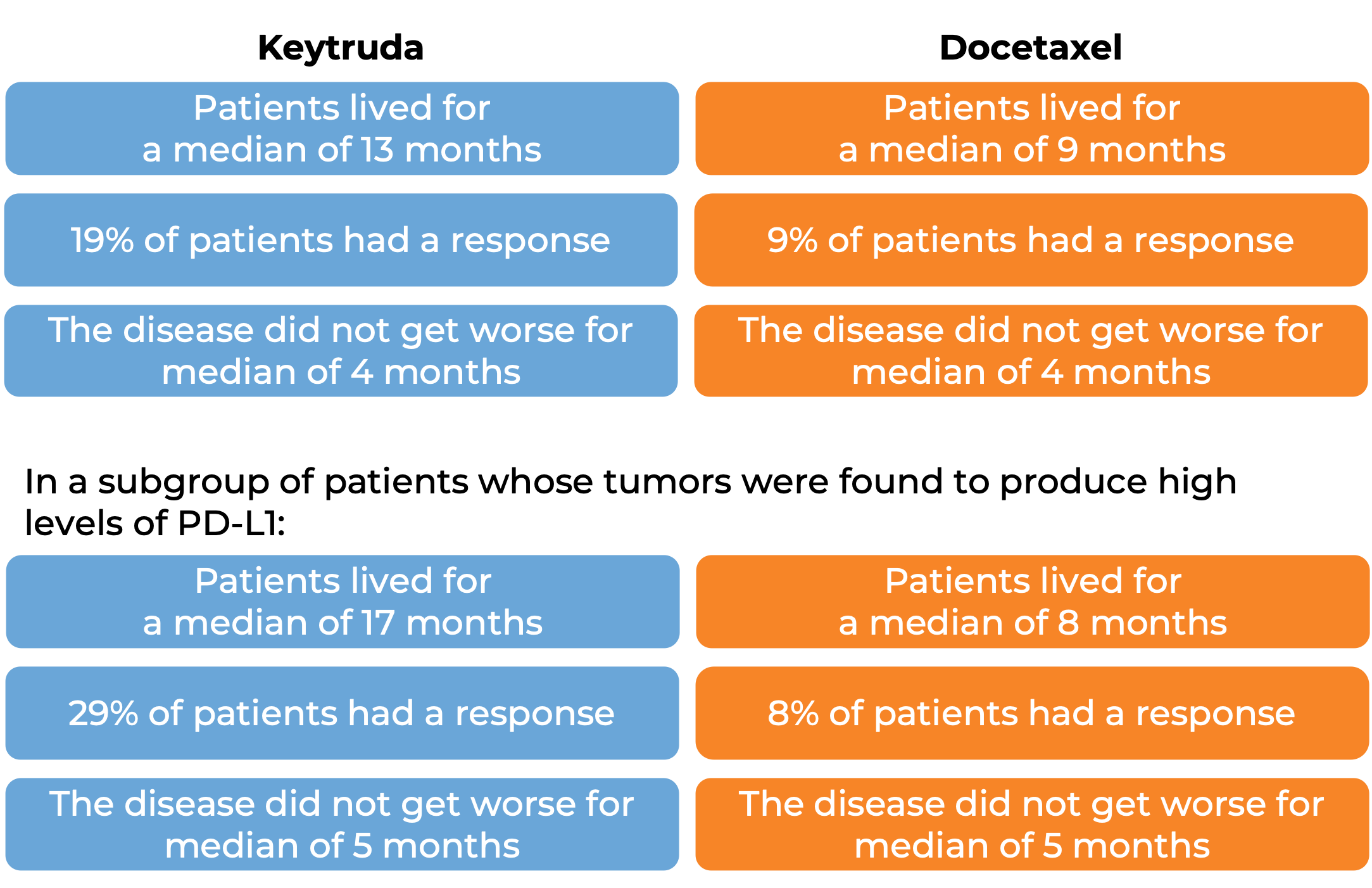
In a clinical trial, 1033 patients with metastatic non-small cell lung cancer that tested positive for PD-L1 who had tried chemotherapy containing platinum, and it either did not work or stopped working were treated either with Keytruda or docetaxel (a chemotherapy). At a median follow-up of 43 months:
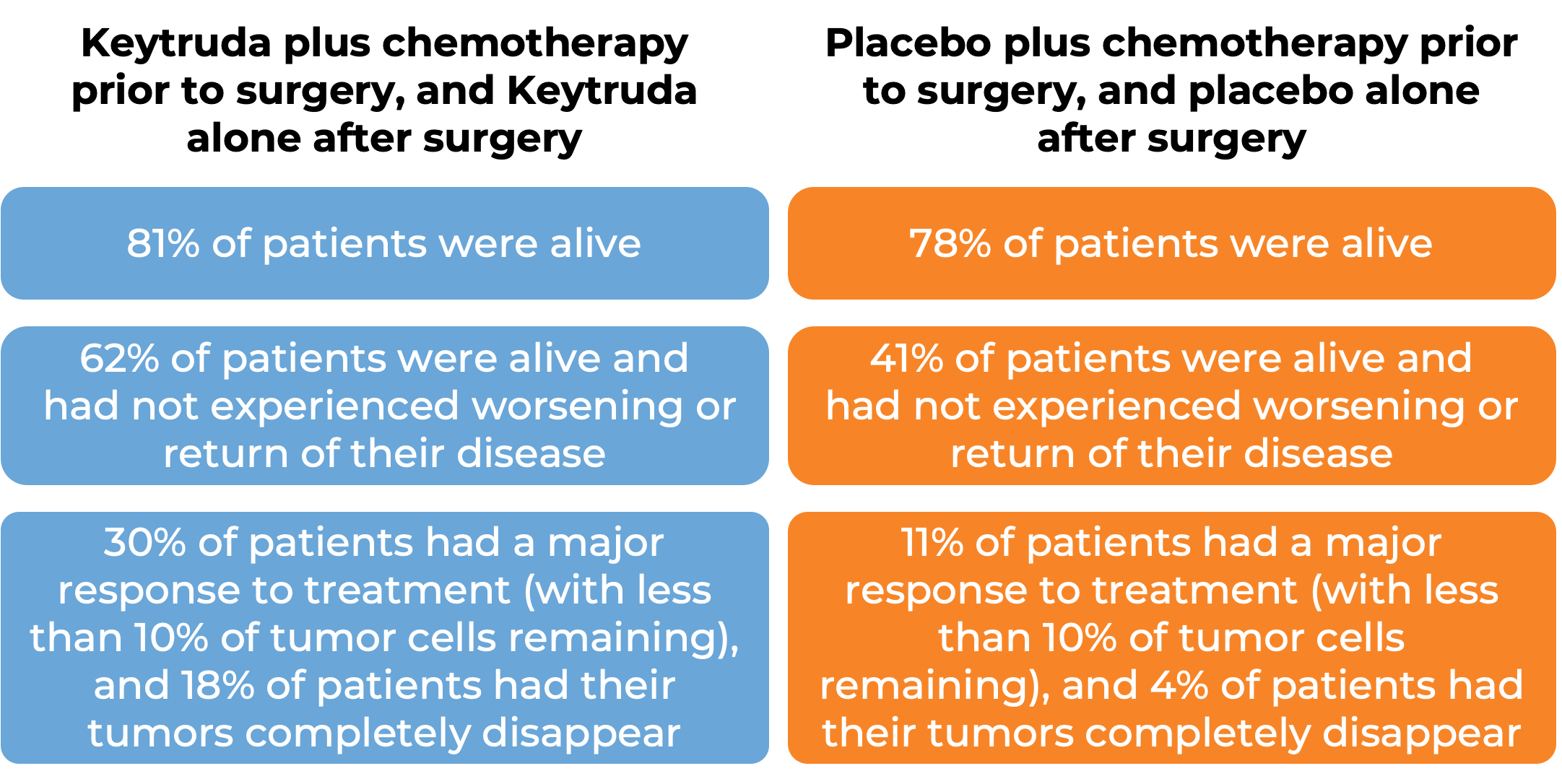
Keytruda for non-small cell lung cancer with chemotherapy before surgical removal and alone after surgical removal
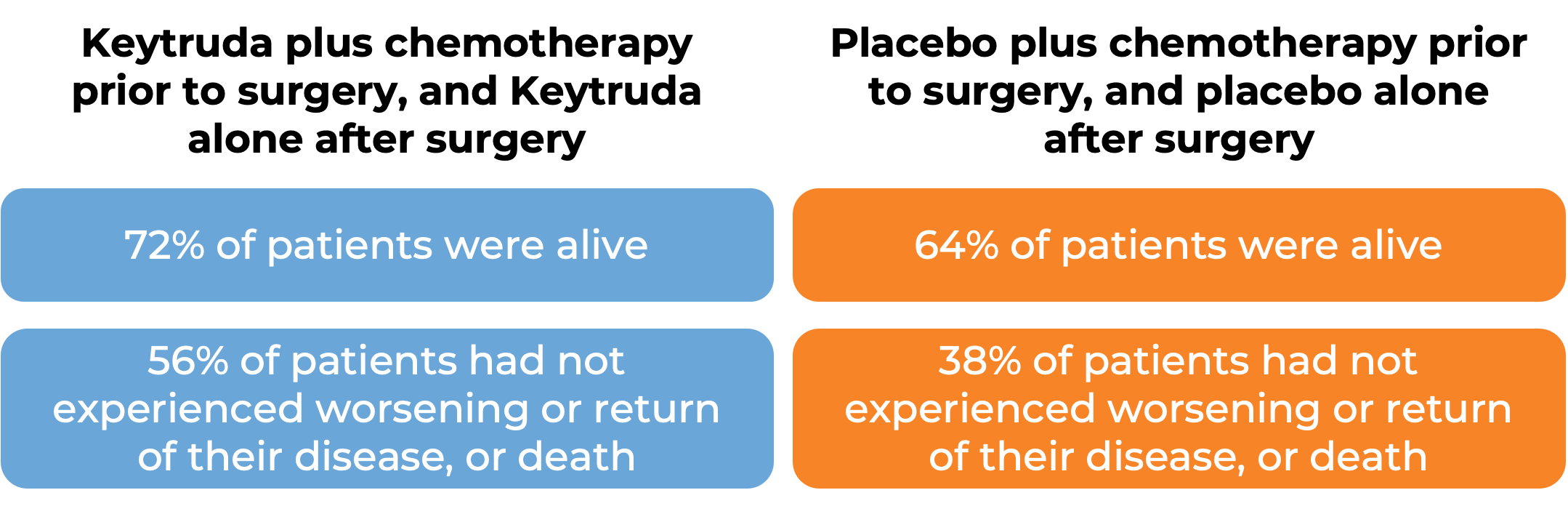
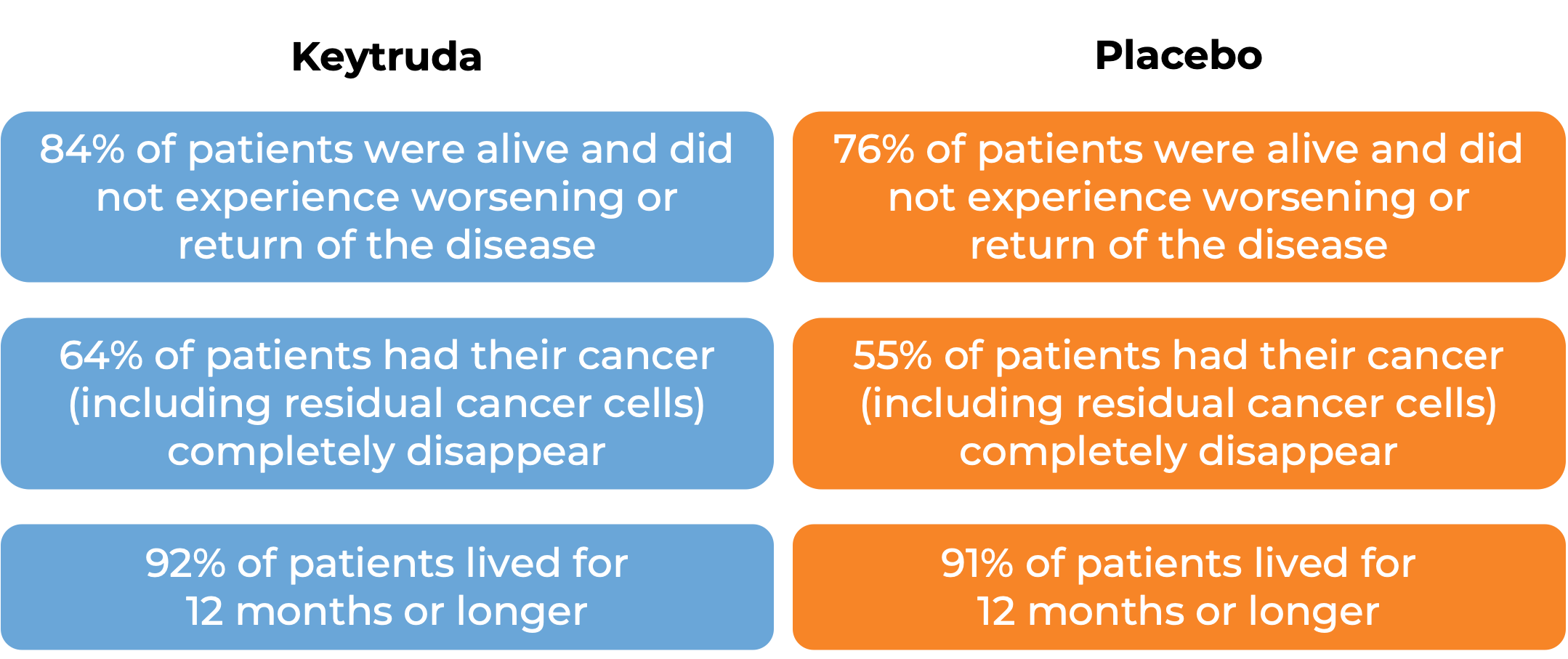
In a clinical trial, 797 patients with previously untreated Stage II, IIIA, or IIIB non-small cell lung cancer that could be removed by surgery (resectable) were treated with either Keytruda plus platinum-based chemotherapy or placebo plus platinum-based chemotherapy prior to surgical removal of the tumor. After surgery, the patients who had received Keytruda continued to receive Keytruda, while those who did not receive Keytruda were treated with a placebo. At a median follow-up of 25 months:
(For the definition of “median”, click HERE.)
Keytruda for non-small cell lung cancer after surgical removal
In a clinical trial, 1010 patients with stage IB, II, or IIIA non-small cell lung cancer whose cancer was removed by surgery and who were treated with a short course of platinum-based chemotherapy following surgery, were treated with either Keytruda or placebo for up to one year.
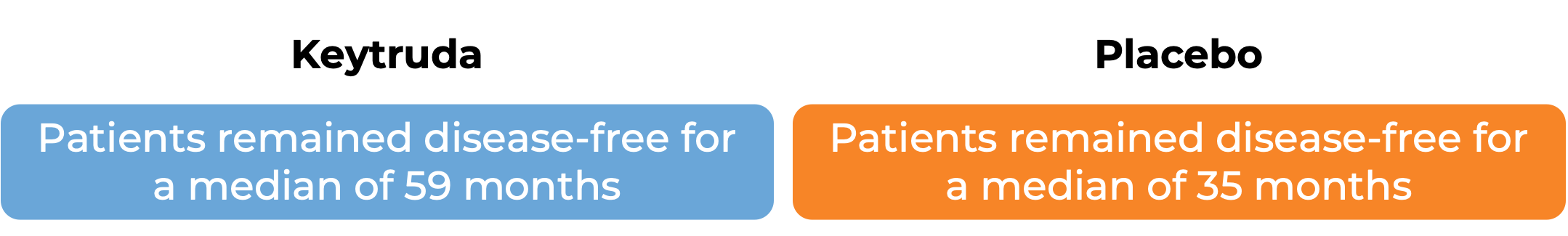
(For the definition of “median”, click HERE.)
Head and neck squamous cell cancer
Keytruda as a first treatment, either alone or with chemotherapy
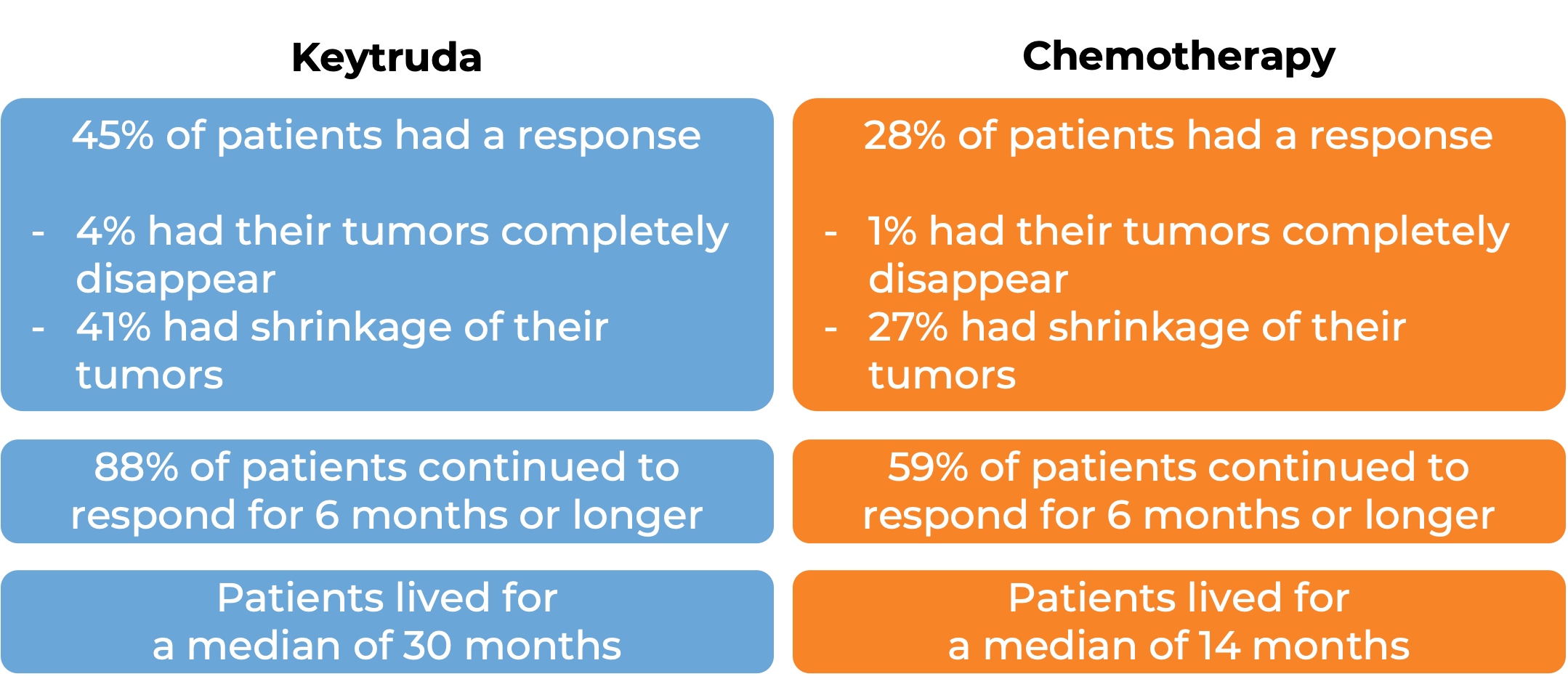
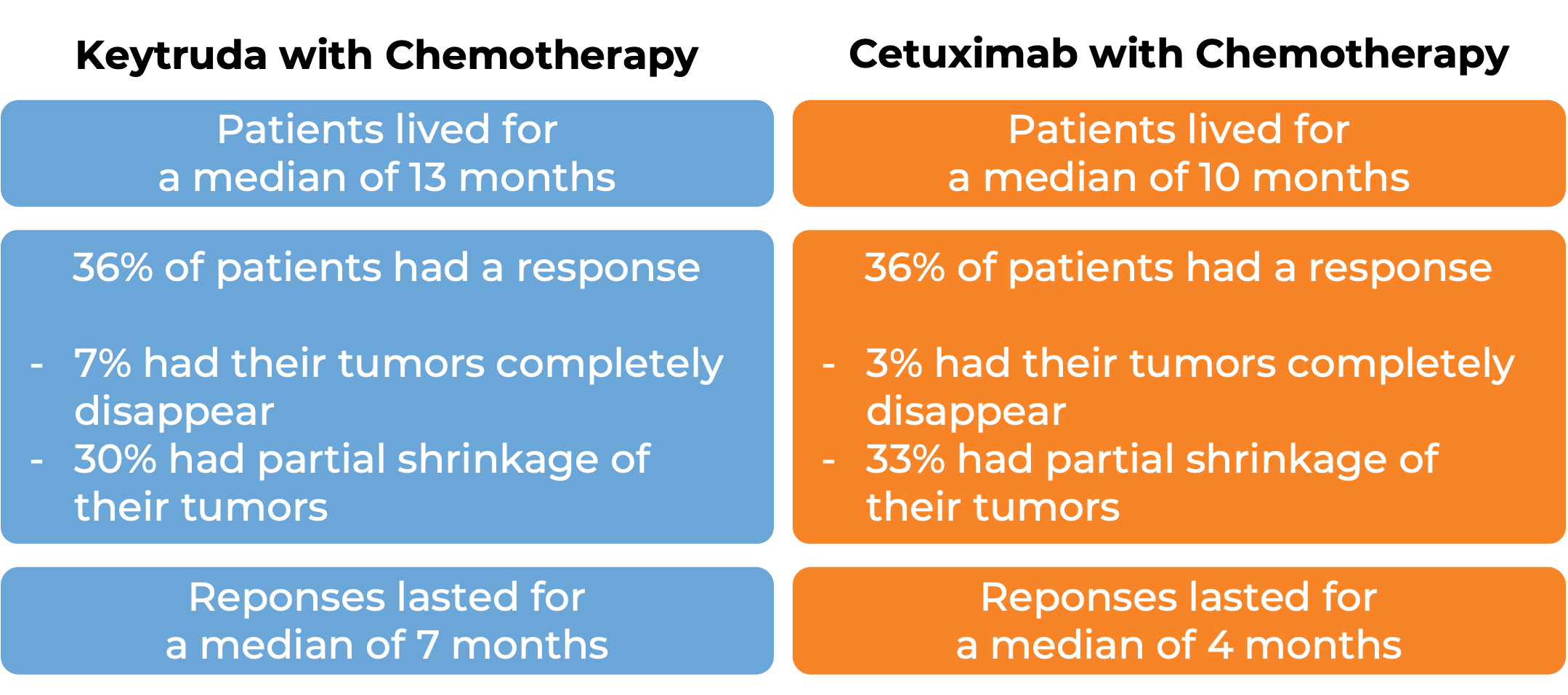
In a clinical trial, 882 patients with head and neck squamous cell cancer that had spread who had not previously received treatment for metastatic disease, or whose cancer came back and could not be removed by surgery were treated with either Keytruda alone, Keytruda with chemotherapy (carboplatin or cisplatin and fluorouracil), or cetuximab (Erbitux) with chemotherapy. At a median follow-up of 13 months: 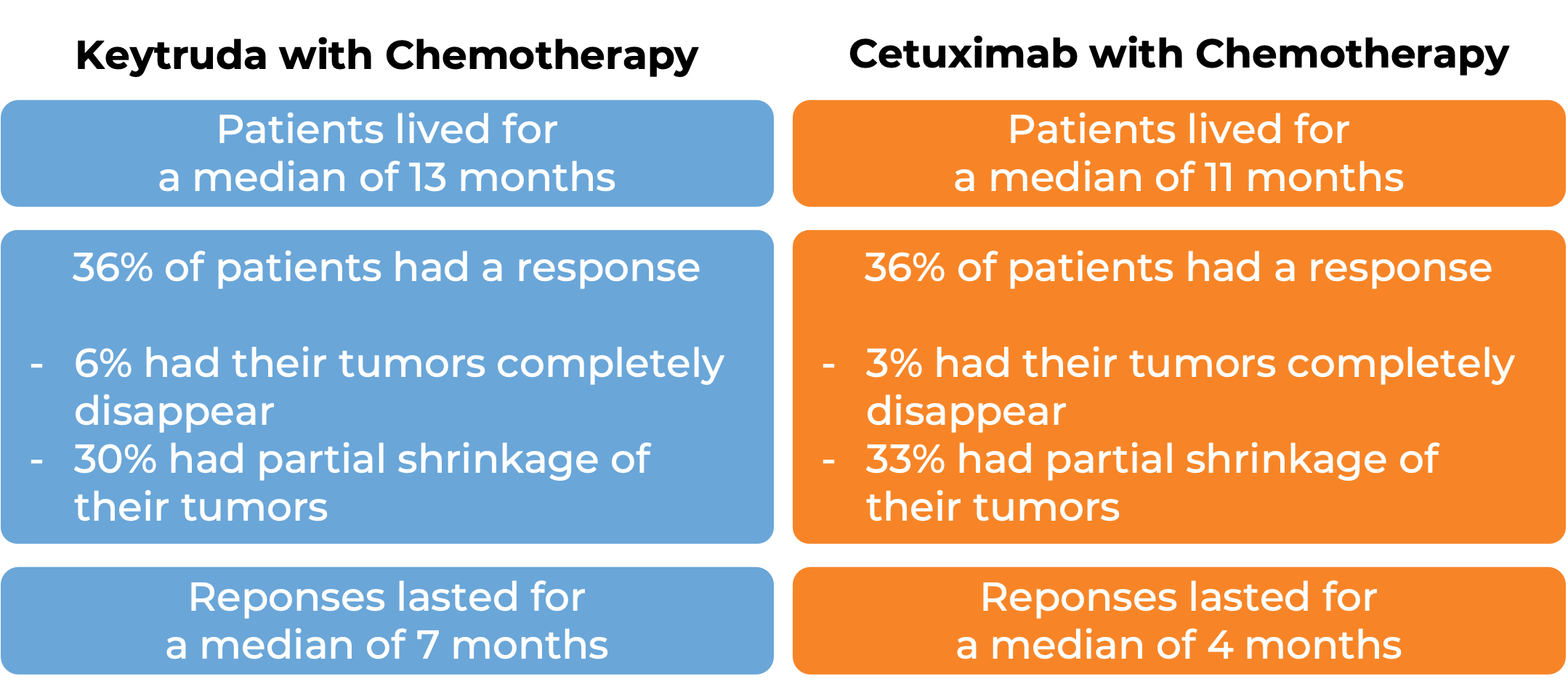
Keytruda for previously treated head and neck squamous cell cancer
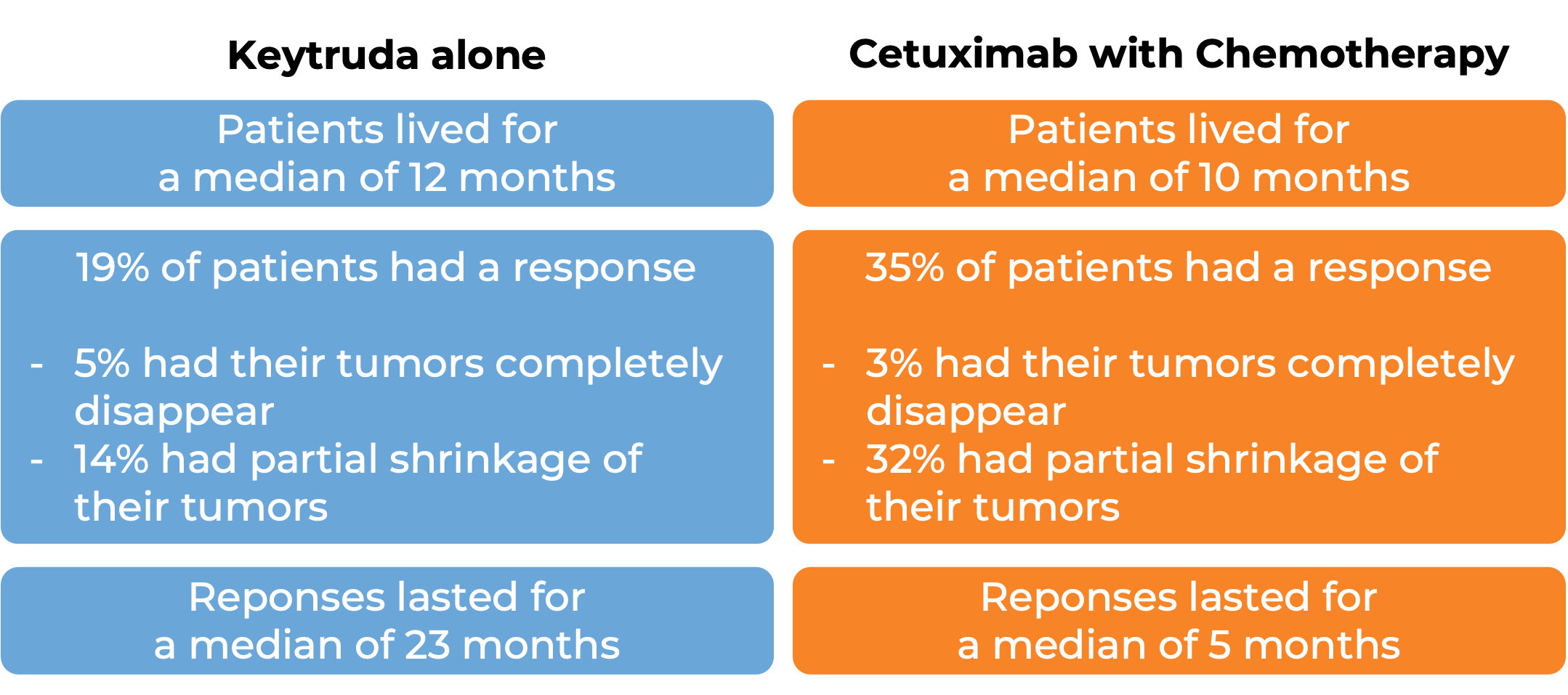
In a clinical trial, 174 patients with head and neck squamous cell carcinoma whose cancer had spread or come back after treatment with chemotherapy that contains platinum were treated with Keytruda. At a median follow-up of 9 months:
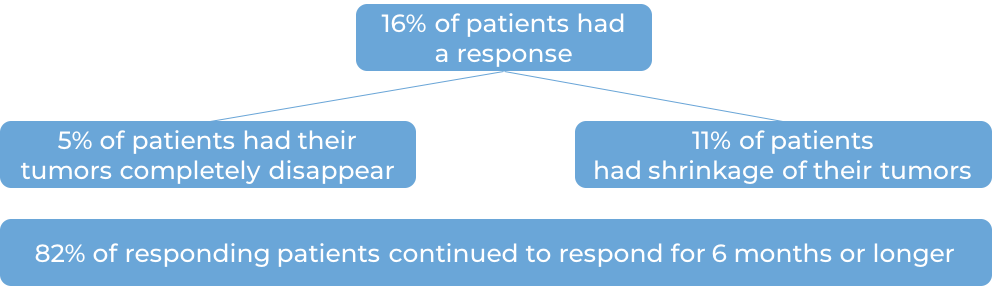
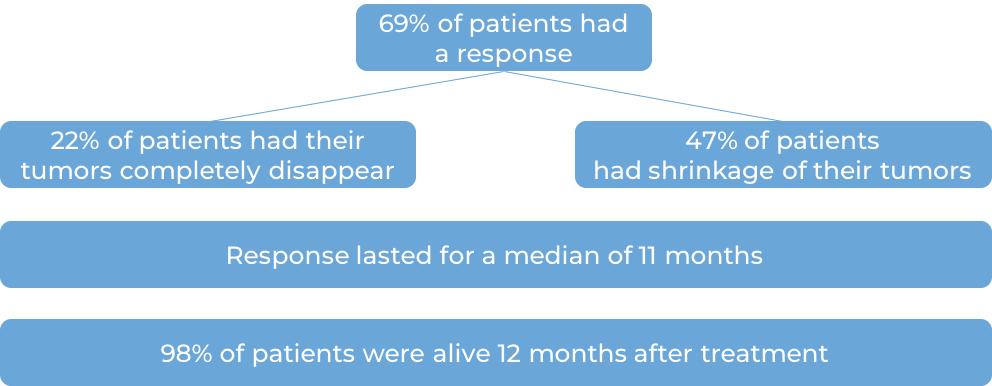
Classical Hodgkin lymphoma
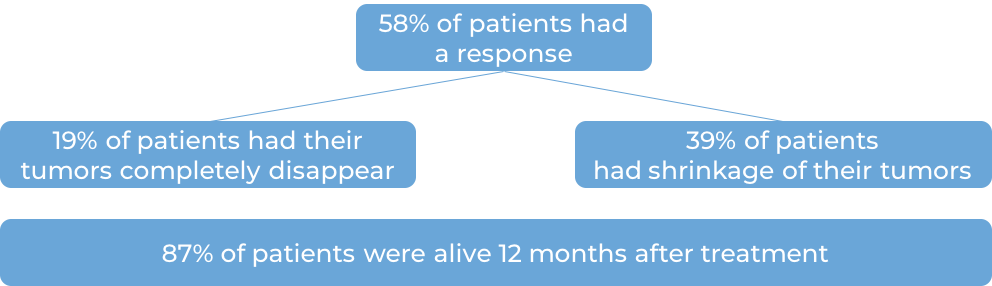
In a clinical trial, 210 patients with classical Hodgkin lymphoma that either had not responded to treatment, or had gotten better, but then came back after treatment were treated with Keytruda. At a median follow-up of 9 months:
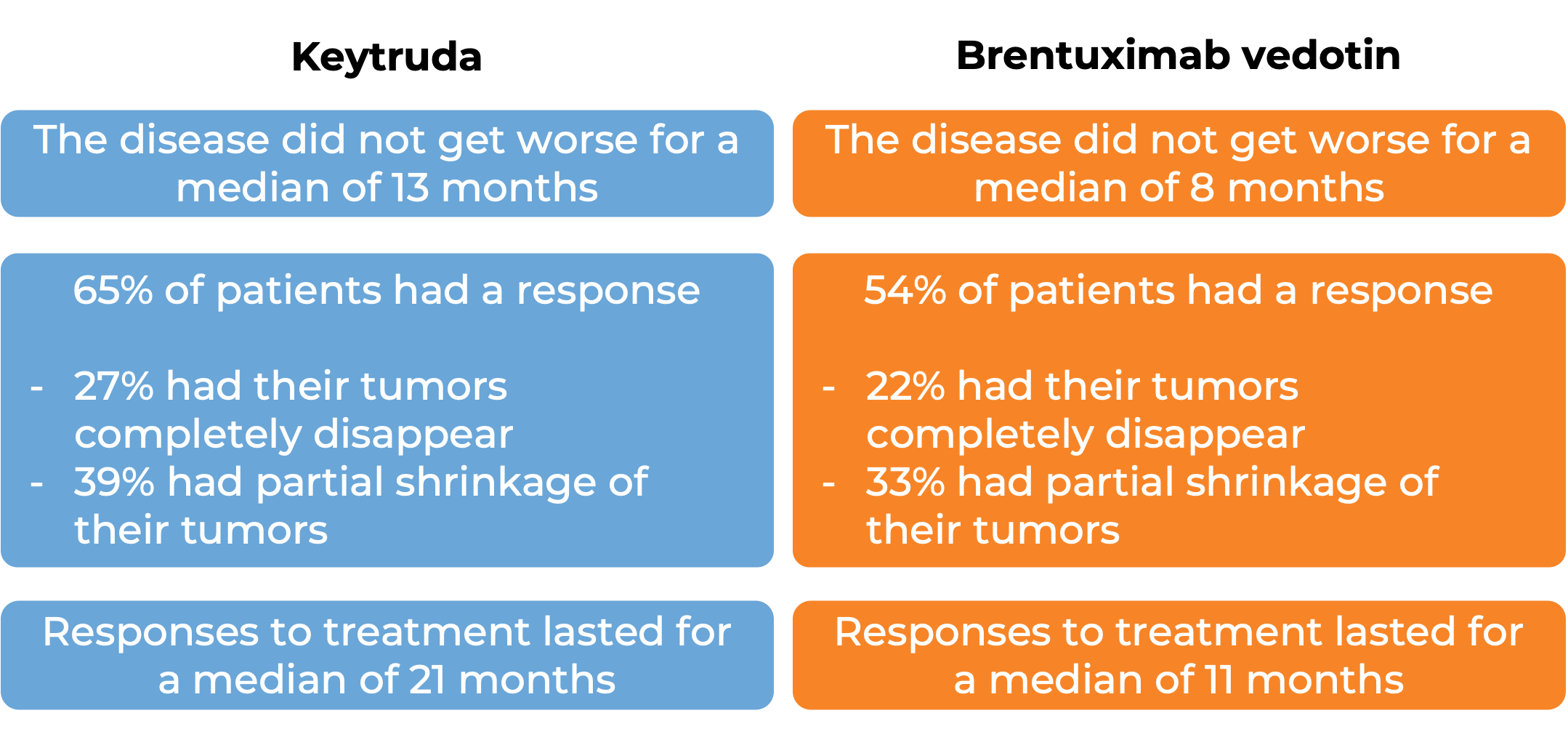
In another clinical trial, 304 adult patients with patients with classical Hodgkin lymphoma that either has not responded to treatment, or got better, but then came back after at least one combination treatment were treated with either Keytruda or brentuximab vedotin. At a median follow-up of 25 months:
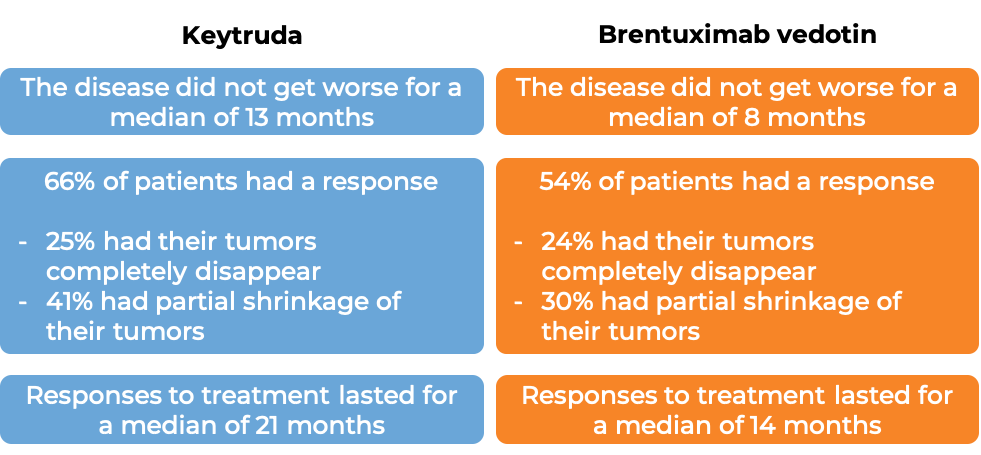
Primary mediastinal B cell lymphoma
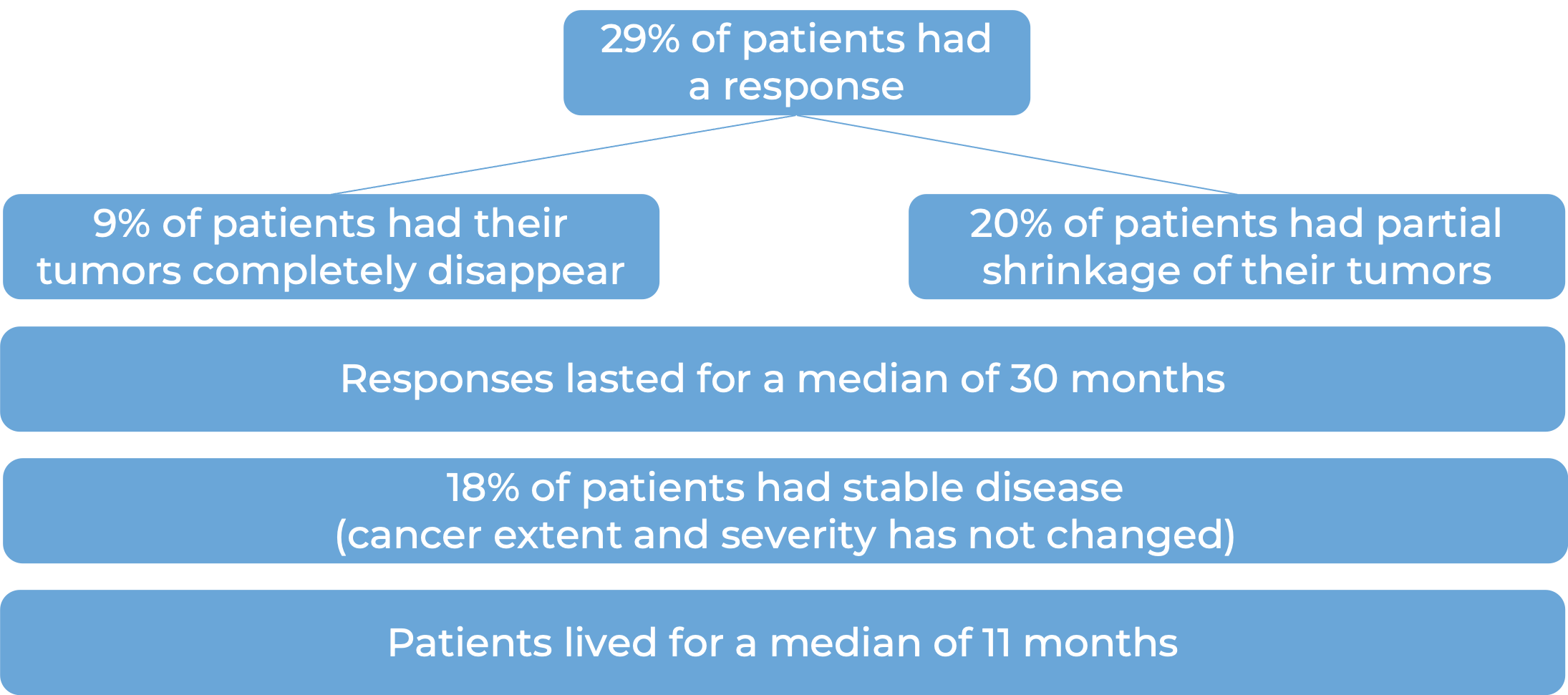
In a clinical trial, 53 patients with primary mediastinal B cell lymphoma that either had not responded to treatment, or had gotten better but then came back after treatment were treated with Keytruda. At a median follow-up of 10 months:
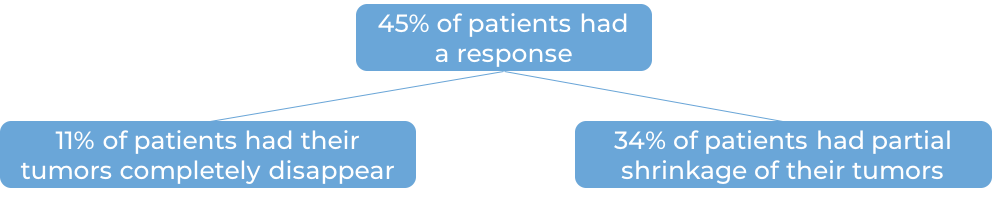
Bladder and urinary tract (urothelial cell) cancer
Keytruda for bladder and urinary tract cancer not suited for platinum-based chemotherapy
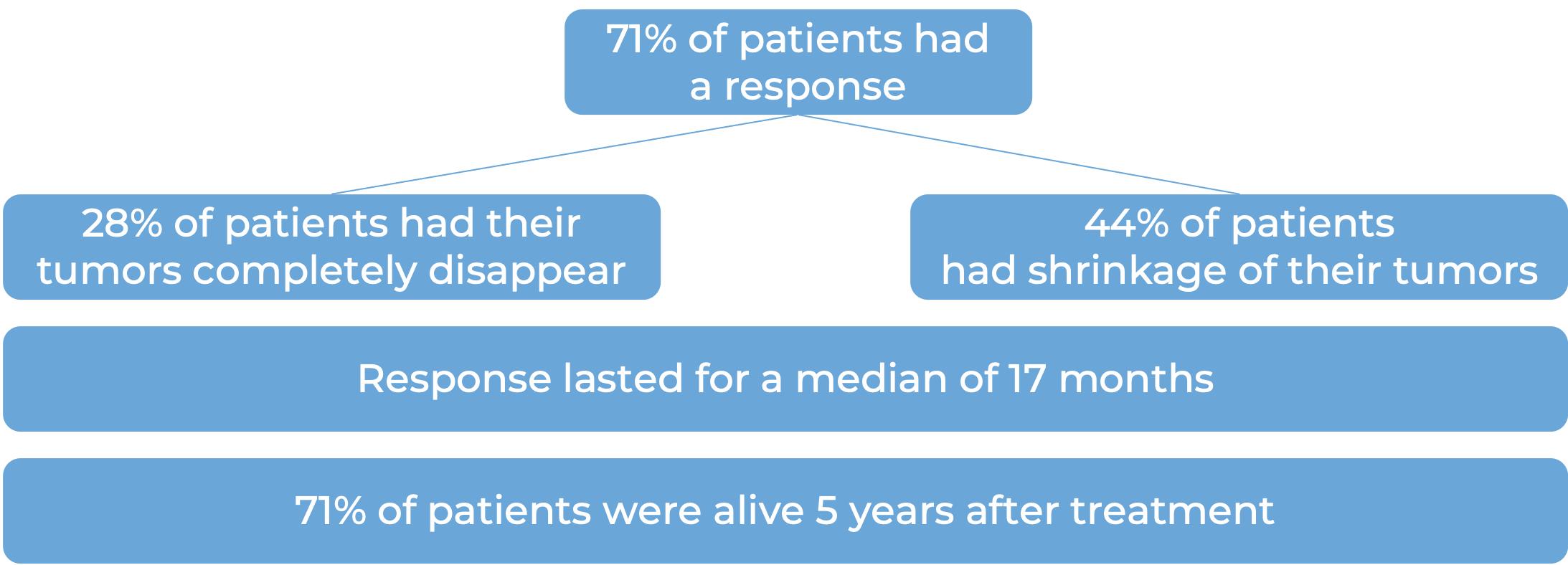
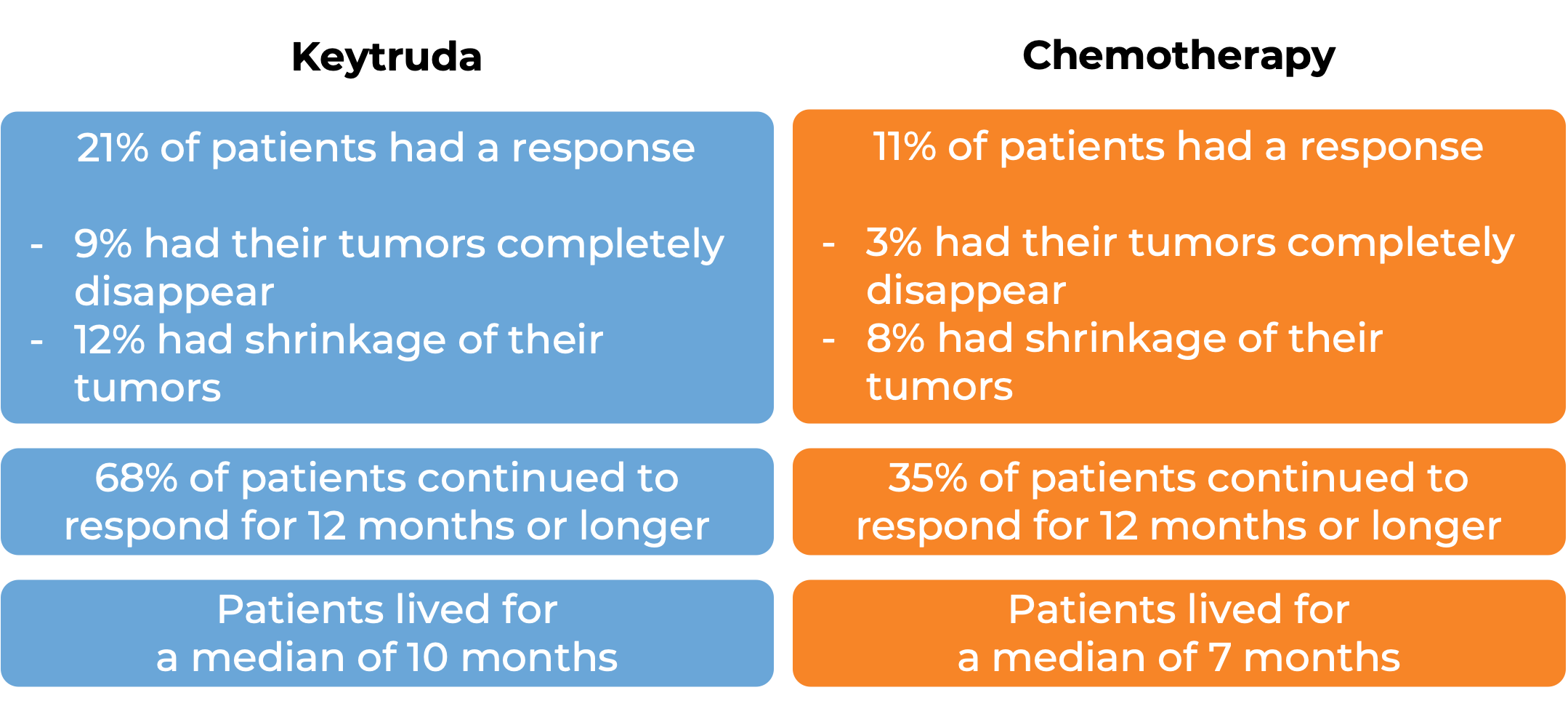
In a clinical trial, 370 patients with advanced urothelial carcinoma that had grown or spread who could not be treated with a platinum-based chemotherapy were treated with Keytruda. At a median follow-up of 11 months:
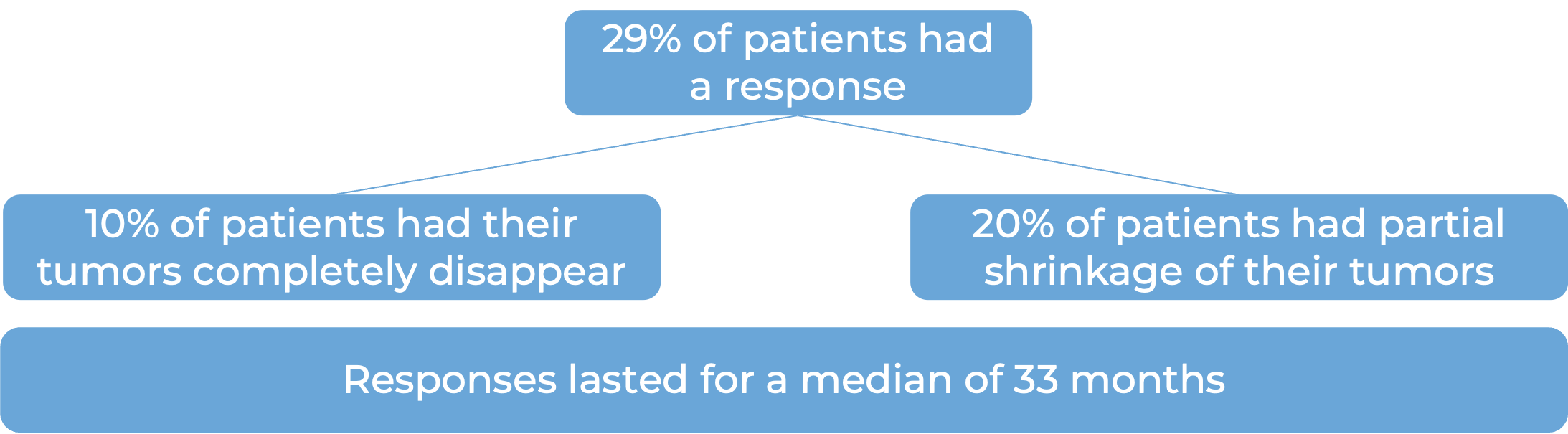
Note: Rounding percentages after the decimal can cause inconsistencies between the total and the sum of the parts.
Keytruda for bladder and urinary tract cancer previously treated with platinum-based chemotherapy
In a clinical trial, 542 patients with advanced urothelial carcinoma that had grown or spread who had been treated with chemotherapy containing platinum, and it did not work or stopped working were treated with Keytruda or chemotherapy (investigator's choice of paclitaxel, docetaxel, or vinflunine). At a median follow-up of 9 months:
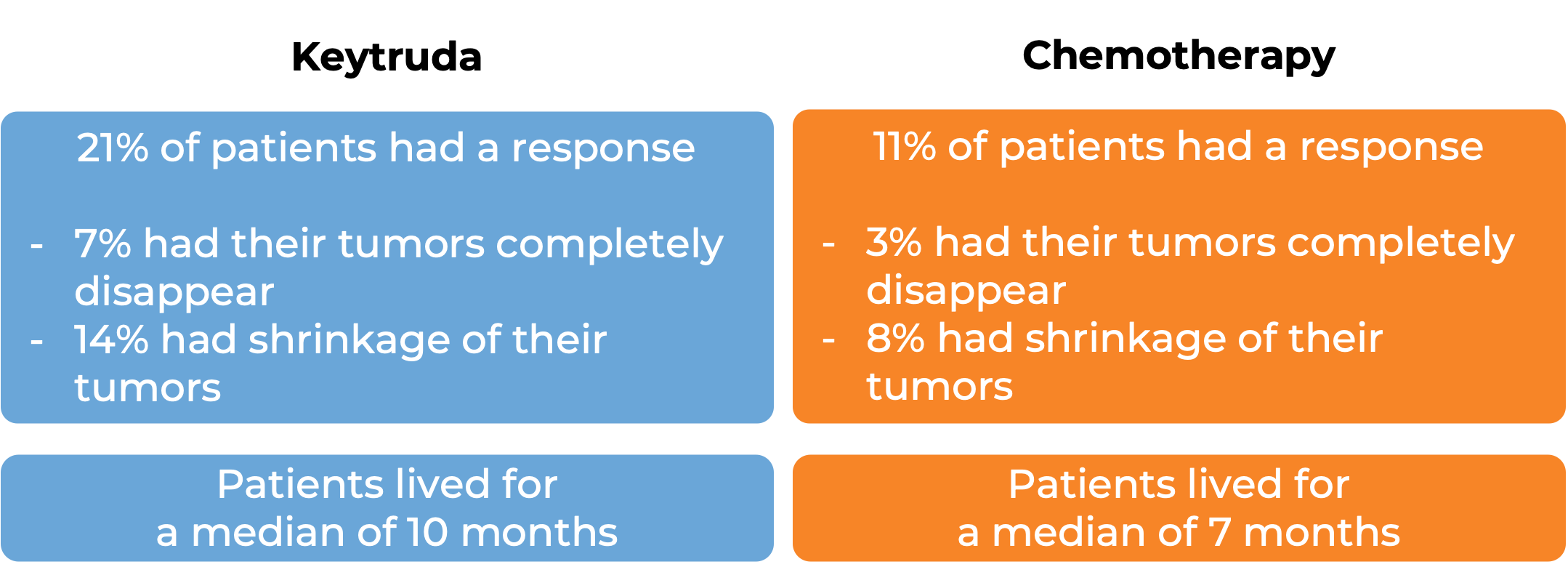
Keytruda for bladder and urinary tract cancer not suited for cisplatin-containing chemotherapy
In a clinical trial, 121 patients with bladder and urinary tract cancer (urothelial cancer) that had grown or spread to other parts of the body, who had not received treatment for their advanced disease and were not suited to receive cisplatin-containing chemotherapy, were treated with Keytruda and enfortumab vedotin (Padcev).
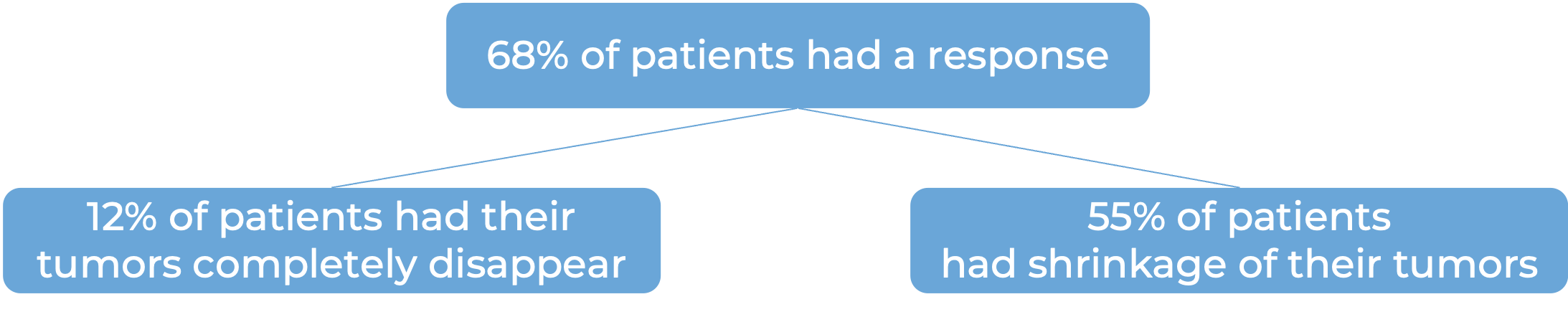
Keytruda in combination with enfortumab vedotin
In a clinical trial, 886 patients with bladder and urinary tract cancer (urothelial cancer) that had grown or spread to other parts of the body, who had not received treatment for their advanced disease, were treated with Keytruda plus enfortumab vedotin ( Padcev) or with standard chemotherapy. At a median follow-up of 17 months:
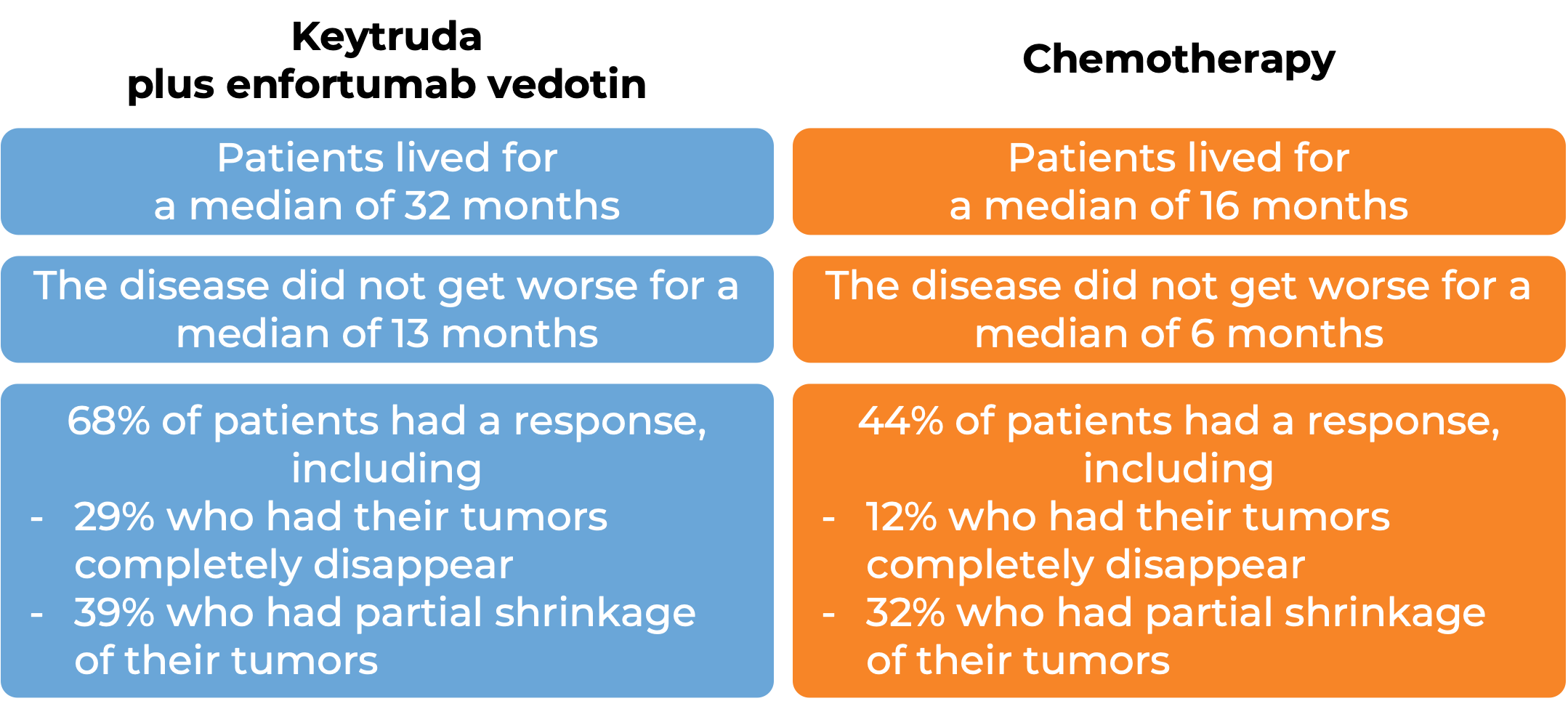
Keytruda for high-risk, non-muscle invasive bladder cancer
In a clinical trial, 96 patients with bladder cancer that had not yet spread to nearby tissue, but was at a high risk for spreading (high-risk, non-muscle invasive bladder cancer), who had tried Bacillus Calmette-Guerin treatment, and it either did not work or stopped working, and who either could not or chose not to have their bladder surgically removed, were treated with Keytruda. At a median follow-up of 28 months, 41% of patients had their tumors completely disappear. Of the patients who responded to treatment, 46% continued to respond for 12 months or longer.
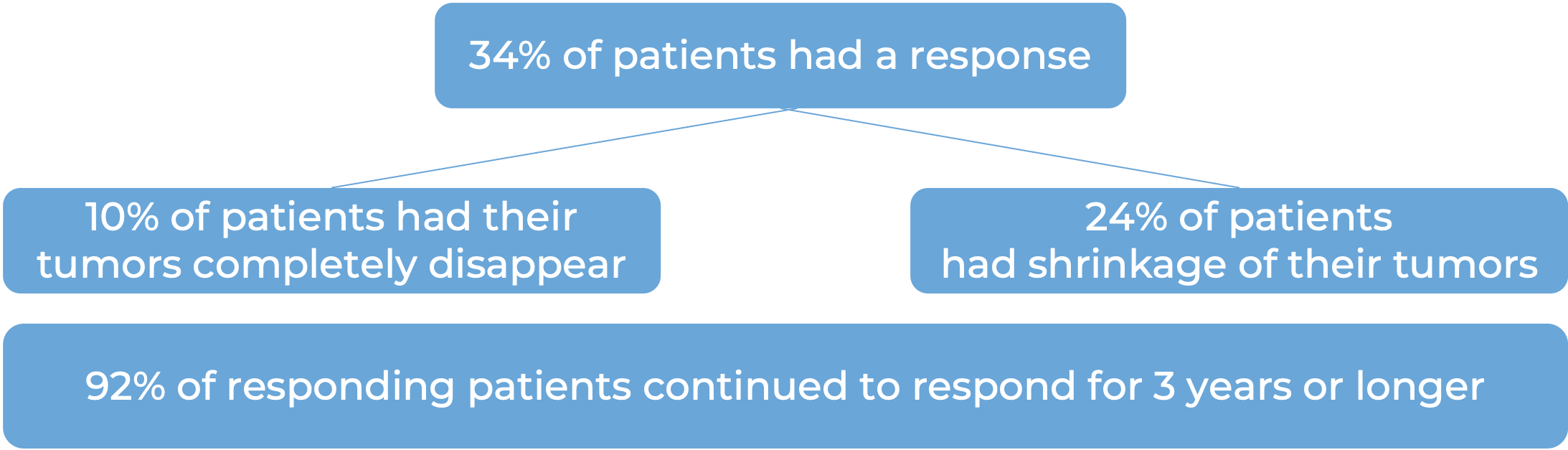
Microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) cancer
Keytruda for previously treated MSI-H or dMMR cancers, including colorectal cancer
A total of 504 patients with microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) solid tumors across three clinical trials were treated with Keytruda. At a median follow-up of 20 months, the following results were observed:
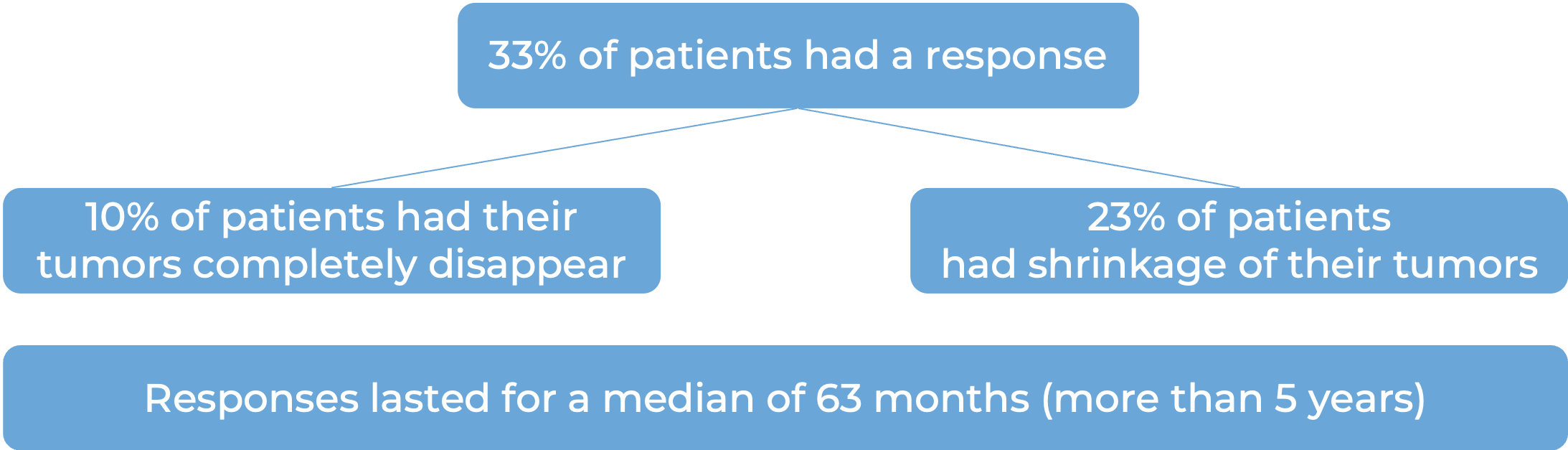
Keytruda for previously untreated MSI-H or dMMR colorectal cancer
In a clinical trial, 307 patients with previously untreated microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) colon or rectal cancer that could not be removed by surgery or had spread to other parts of the body received either Keytruda or the investigator’s choice of the following chemotherapies:
- mFOLFOX6 (oxaliplatin, leucovorin, and fluorouracil)
- mFOLFOX6 (oxaliplatin, leucovorin, and fluorouracil) in combination with either bevacizumab (Avastin, Mvasi, Zirabev) or cetuximab (Erbitux)
- FOLFIRI (irinotecan, leucovorin, and fluorouracil)
- FOLFIRI (irinotecan, leucovorin, and fluorouracil) in combination with either bevacizumab (Avastin, Mvasi, Zirabev) or cetuximab (Erbitux)
At a median follow-up of 38 months:
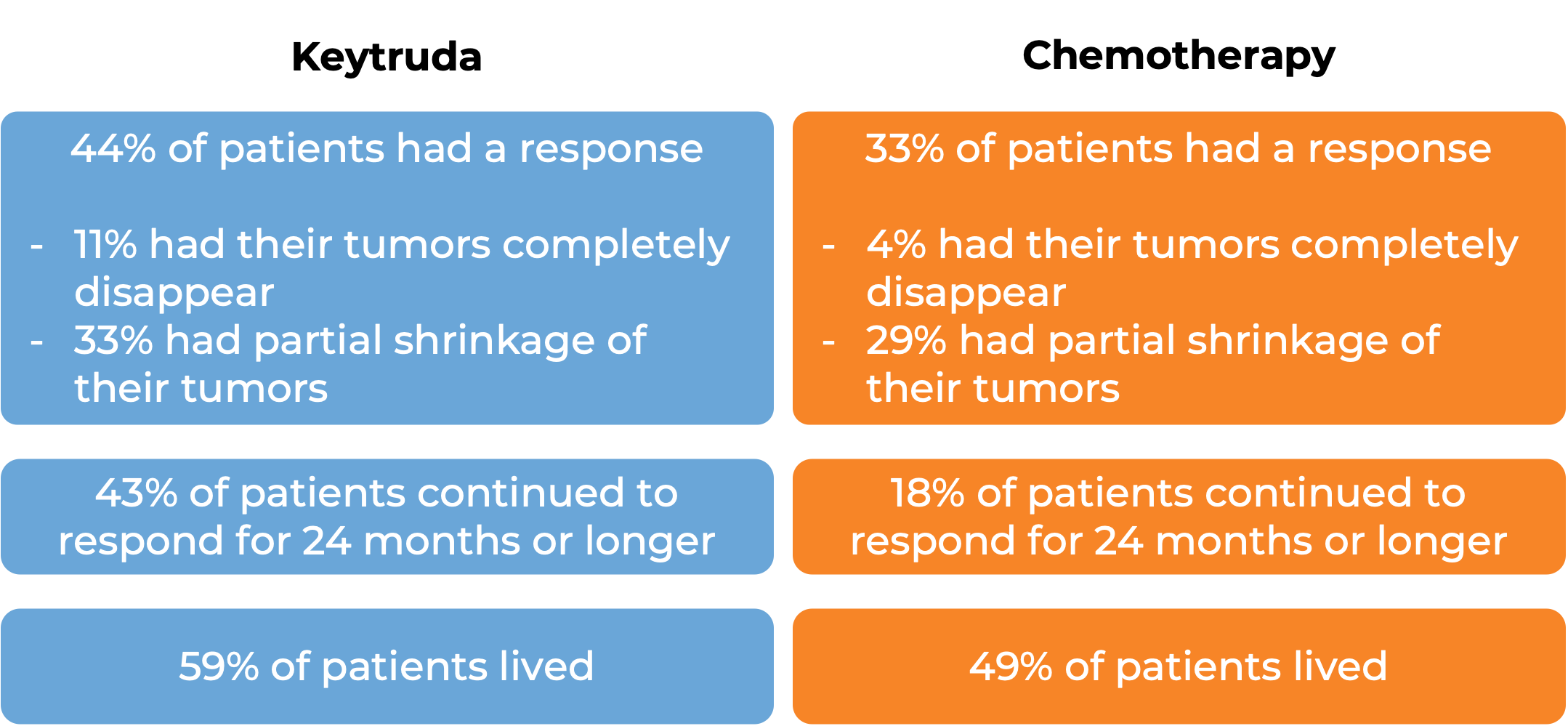
Advanced stomach cancer
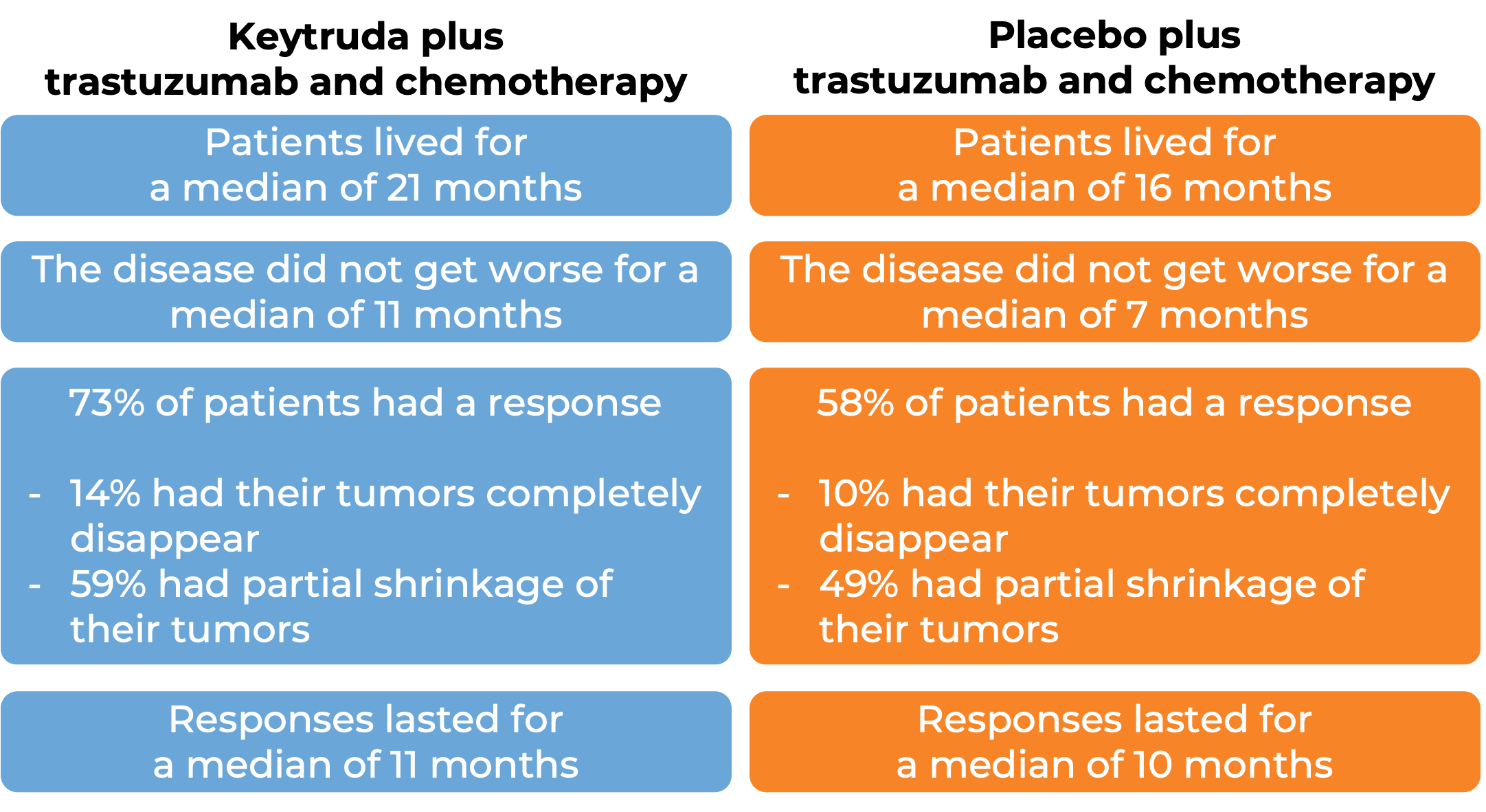
Keytruda for gastric or gastroesophageal junction adenocarcinoma that tests positive for HER2
In a clinical trial, 698 patients with previously untreated gastric or gastroesophageal junction adenocarcinoma that was either locally advanced or had spread to other parts of the body AND tested positive for the HER2 molecule were treated with either:
- Keytruda plus trastuzumab (Herceptin) and chemotherapy, OR
- Placebo plus trastuzumab and chemotherapy.
In an early analysis of 264 patients:
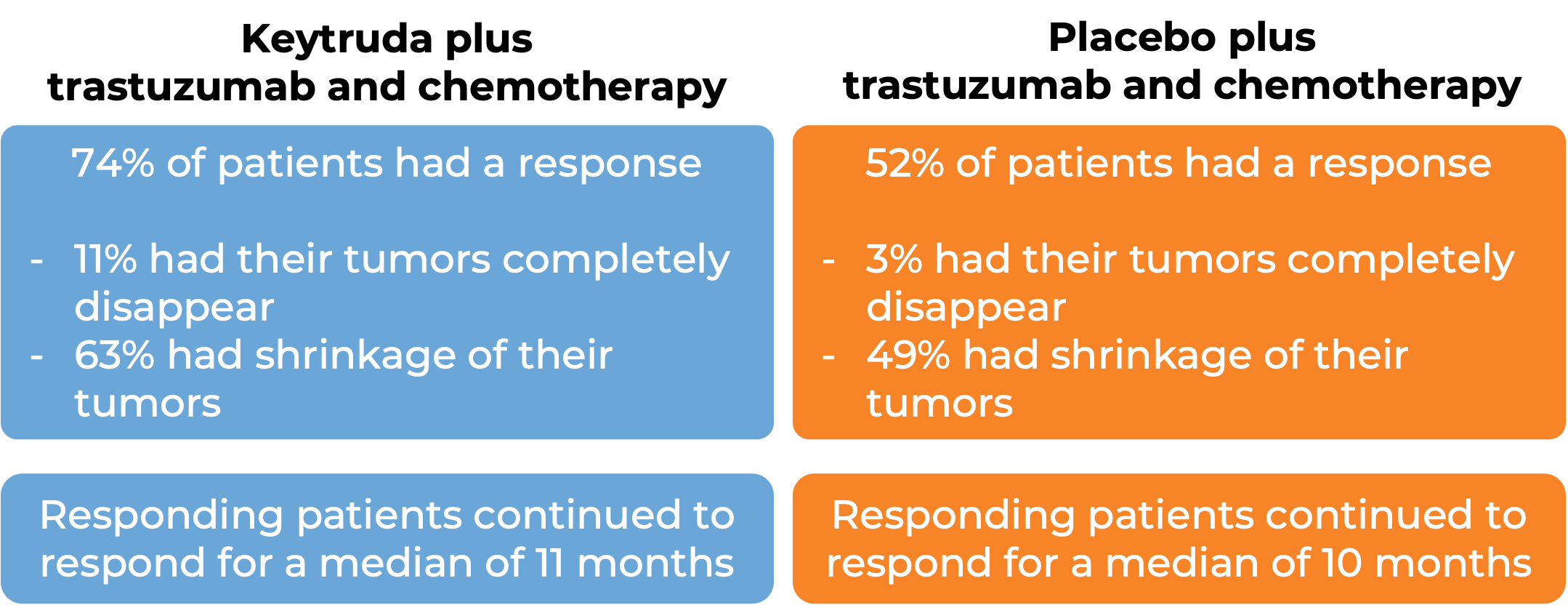
(For the definition of "median" click HERE.)
In later analyses, researchers found that patients whose cancer tested positive for the PD-L1 molecule were likely to benefit from adding Keytruda to trastuzumab and chemotherapy, while those who tested negative saw little improvement over trastuzumab and chemotherapy alone.
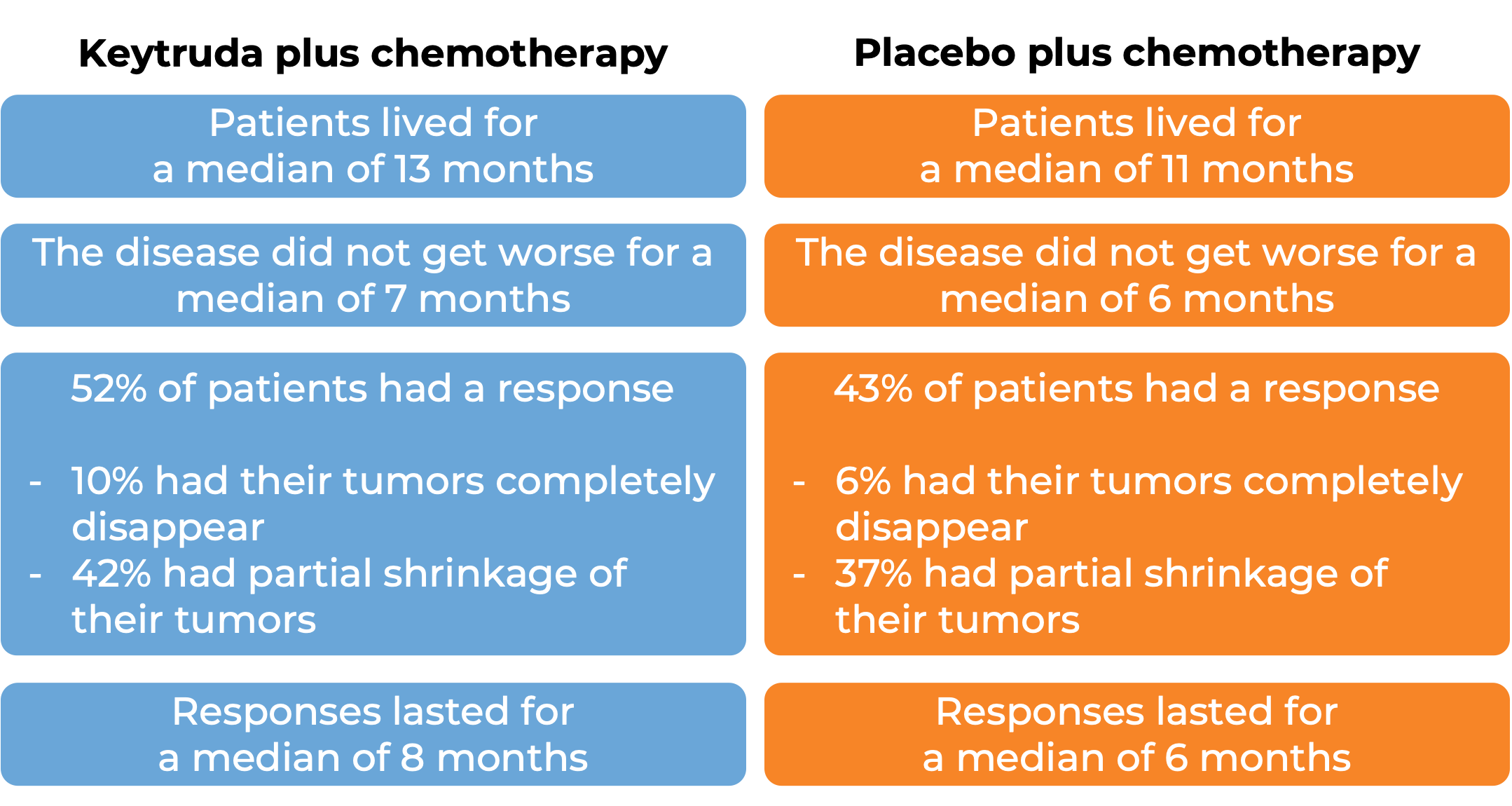
Keytruda for gastric or gastroesophageal junction adenocarcinoma that tests negative for HER2
In a clinical trial, 1,579 patients with gastric or gastroesophageal junction adenocarcinoma that was either locally advanced and could not be removed by surgery or that had spread to other parts of the body, who had not yet received treatment for their advanced disease and whose cancer tested negative for the HER2 molecule, were treated with either Keytruda plus chemotherapy or placebo plus chemotherapy. At a median follow-up of 31 months:
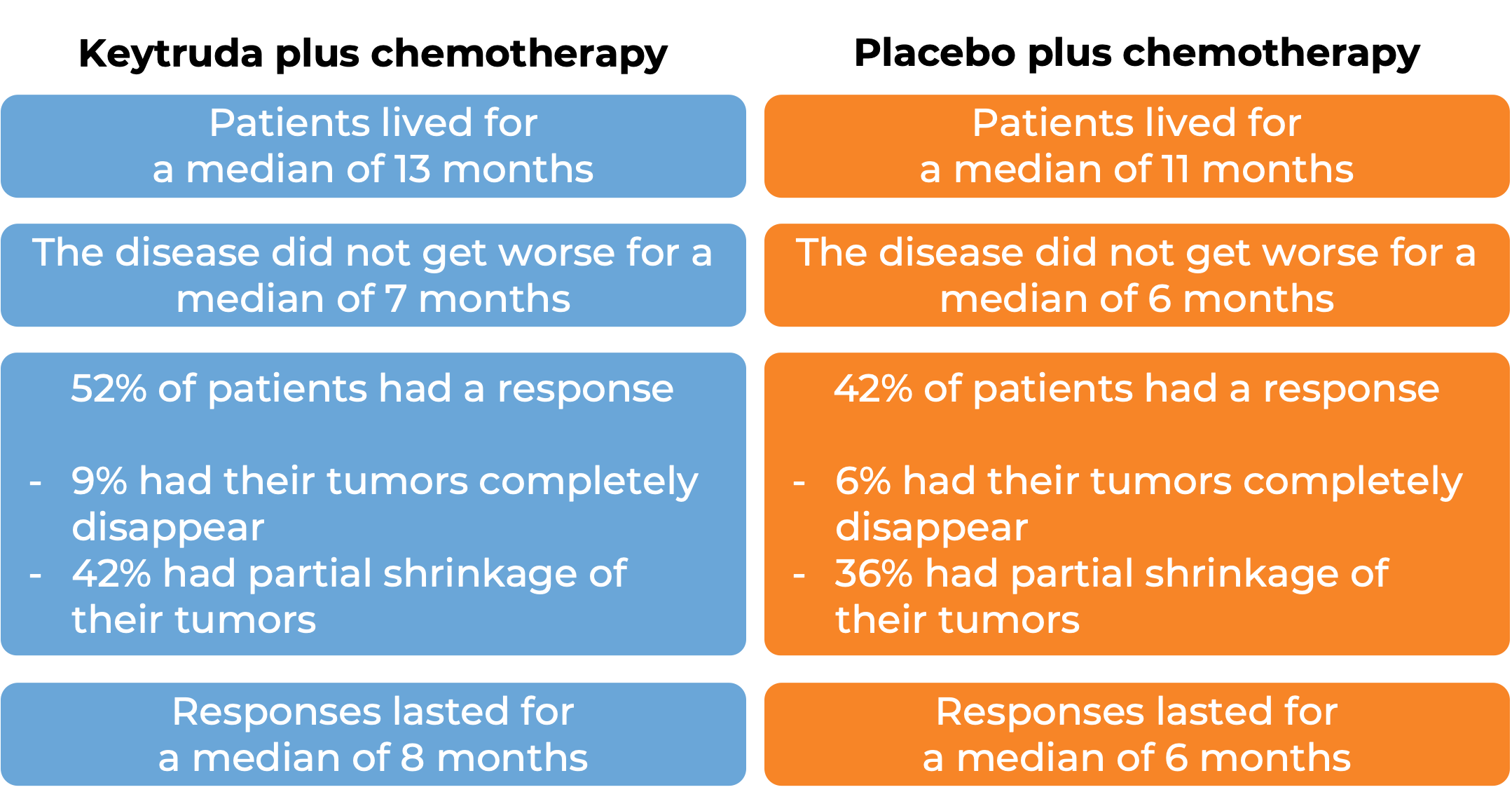
In 551 patients whose tumors test positive for high levels of the PD-L1 moledule:
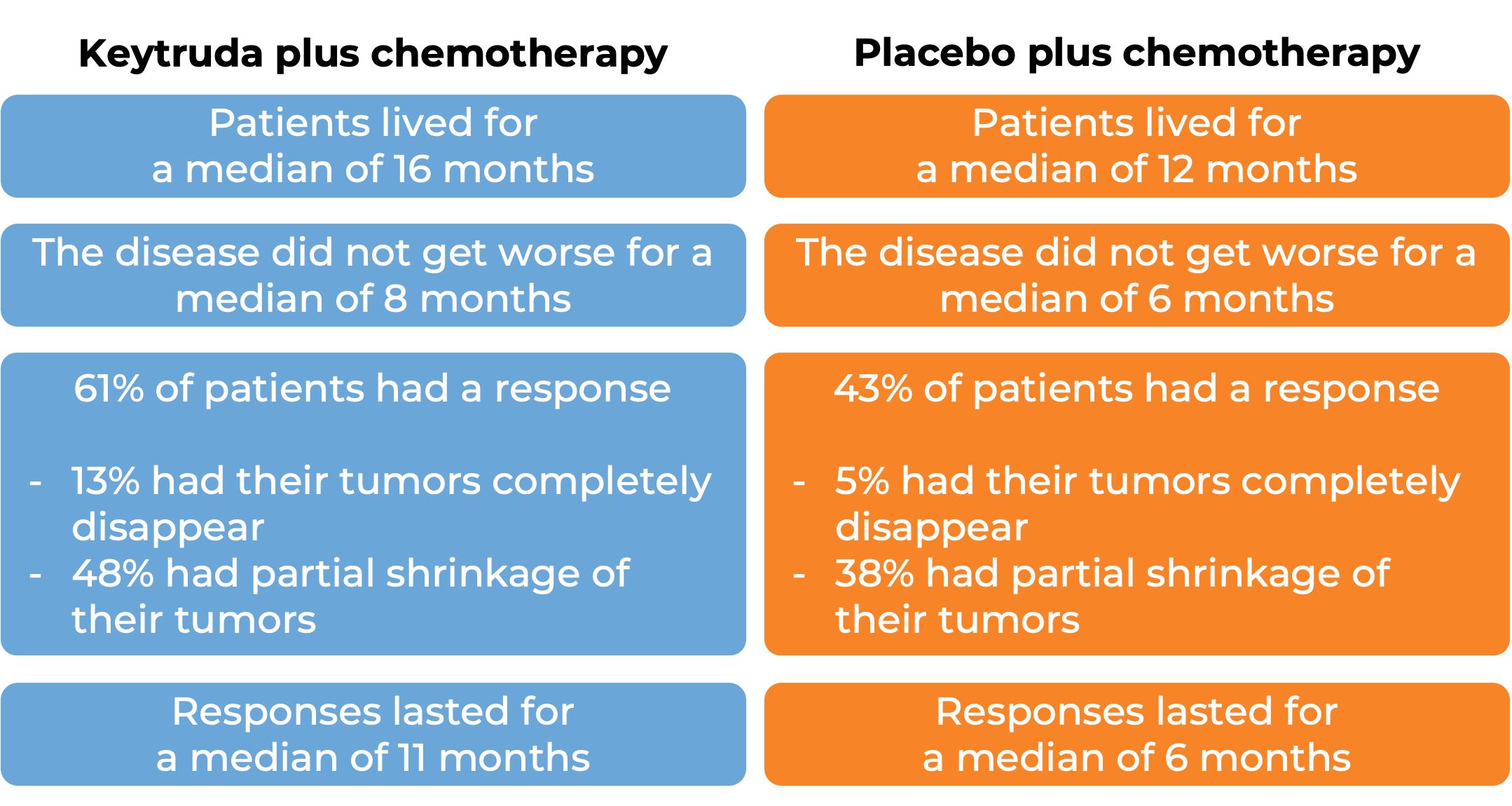
Advanced esophagus cancer
In a clinical trial, 628 patients with esophageal cancer that had come back or spread who had received at least one treatment, and it either did not work or stopped working were treated with Keytruda or chemotherapy (paclitaxel, docetaxel, or irinotecan). For the 167 patients whose esophageal squamous cell carcinoma tested positive for high levels of PD-L1:
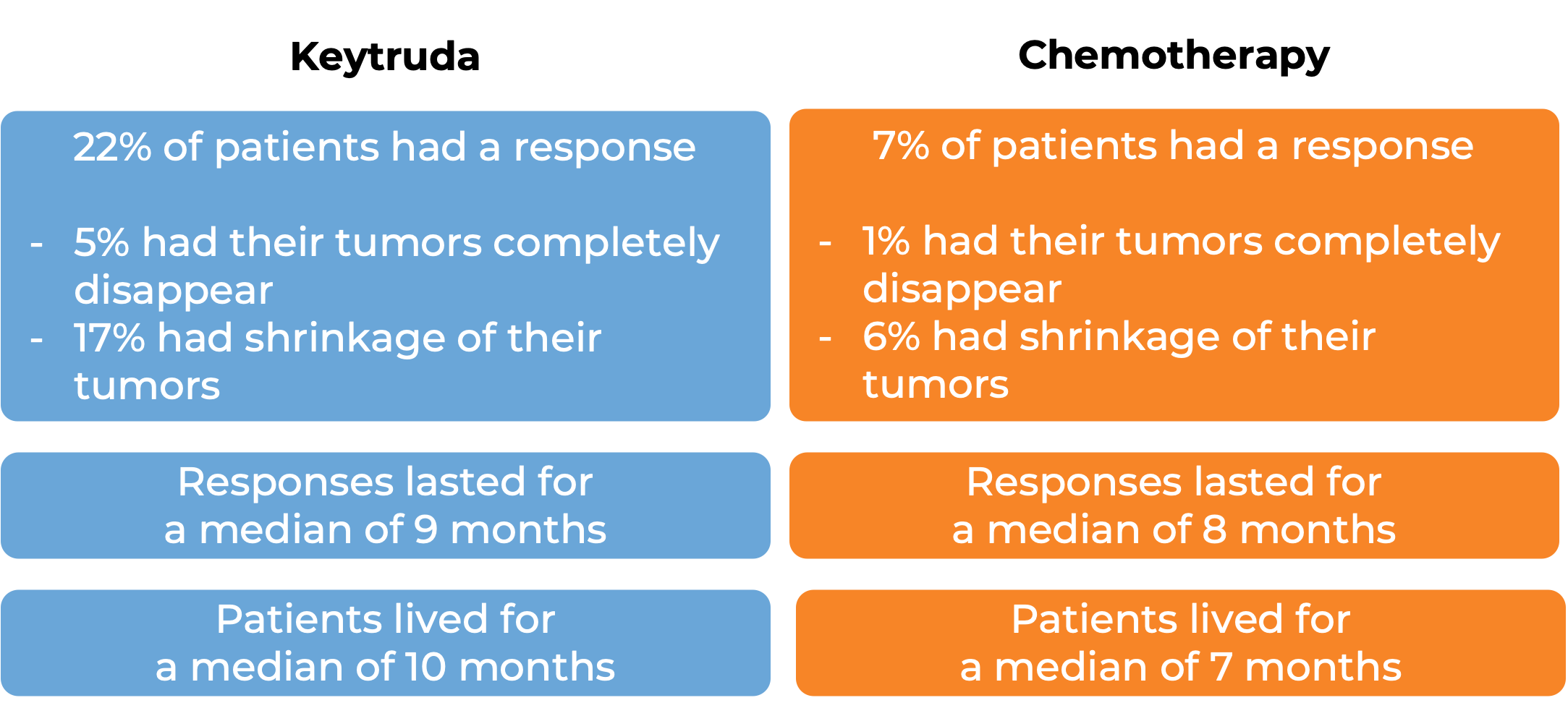
In another clinical trial, 121 patients with esophageal squamous cell carcinoma that had grown locally or spread, and who had received at least two treatments, and they either did not work or stopped working, were treated with Keytruda. Among the 35 patients with cancers that tested positive for PD-L1:

In another clinical trial, 749 patients with esophageal cancer that had spread to nearby lymph nodes or other parts of the body and could not be removed by surgery or treated with concurrent chemotherapy and radiation, were treated with Keytruda plus cisplatin and fluoropyrimidine chemotherapy, or with placebo plus chemotherapy for up to 2 years. At a median follow-up of 23 months:
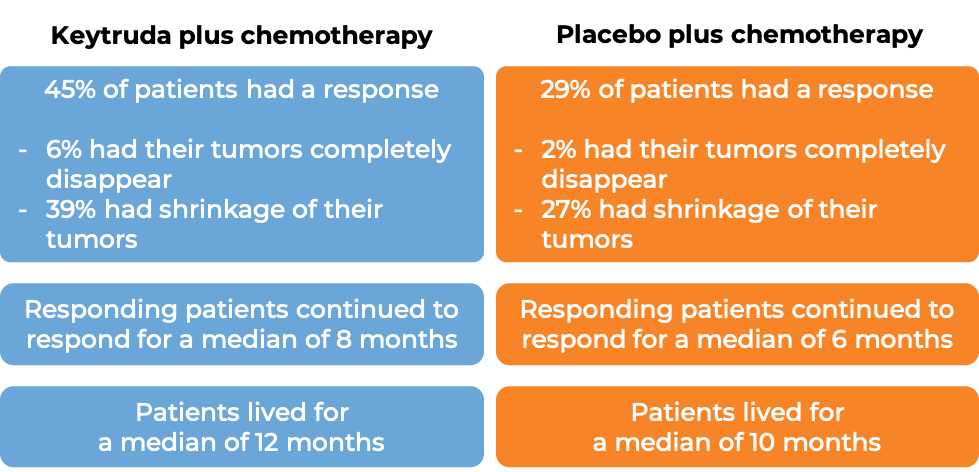
(For the definition of “median”, click HERE.)
Advanced cervical cancer
Keytruda for FIGO 2014 Stage III-IVA cervical cancer
In a clinical trial, 596 patients with FIGO 2014 Stage III-IVA cervical cancer, were treated with either
- Keytruda plus chemotherapy and radiotherapy (CRT), followed by Keytruda alone, OR
- Placebo plus chemotherapy and radiotherapy, followed by placebo alone.
At 12 months, 81% of the patients who received Keytruda plus CRT and 70% of the patients who were treated with placebo plus CRT were alive and did not experience worsening of their cancer.
Keytruda for previously treated advanced cervical cancer
In a clinical trial, 98 patients with cervical cancer that had come back or spread were treated with Keytruda for up to 2 years. Among the 77 patients whose cancers tested positive for PD-L1 and who had prior treatment for their advanced disease, at a median follow-up time of 12 months:
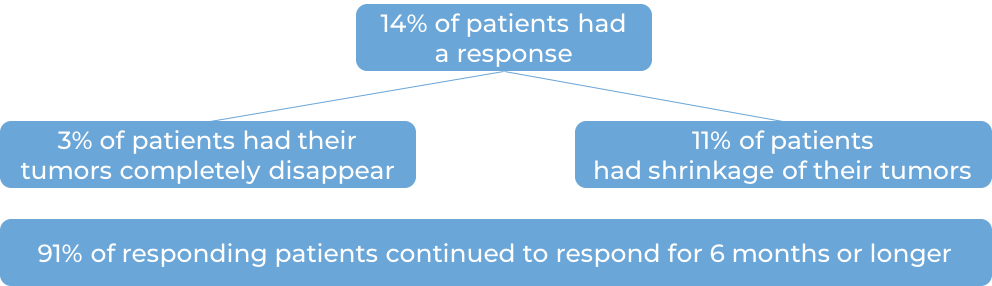
Keytruda for previously untreated advanced cervical cancer
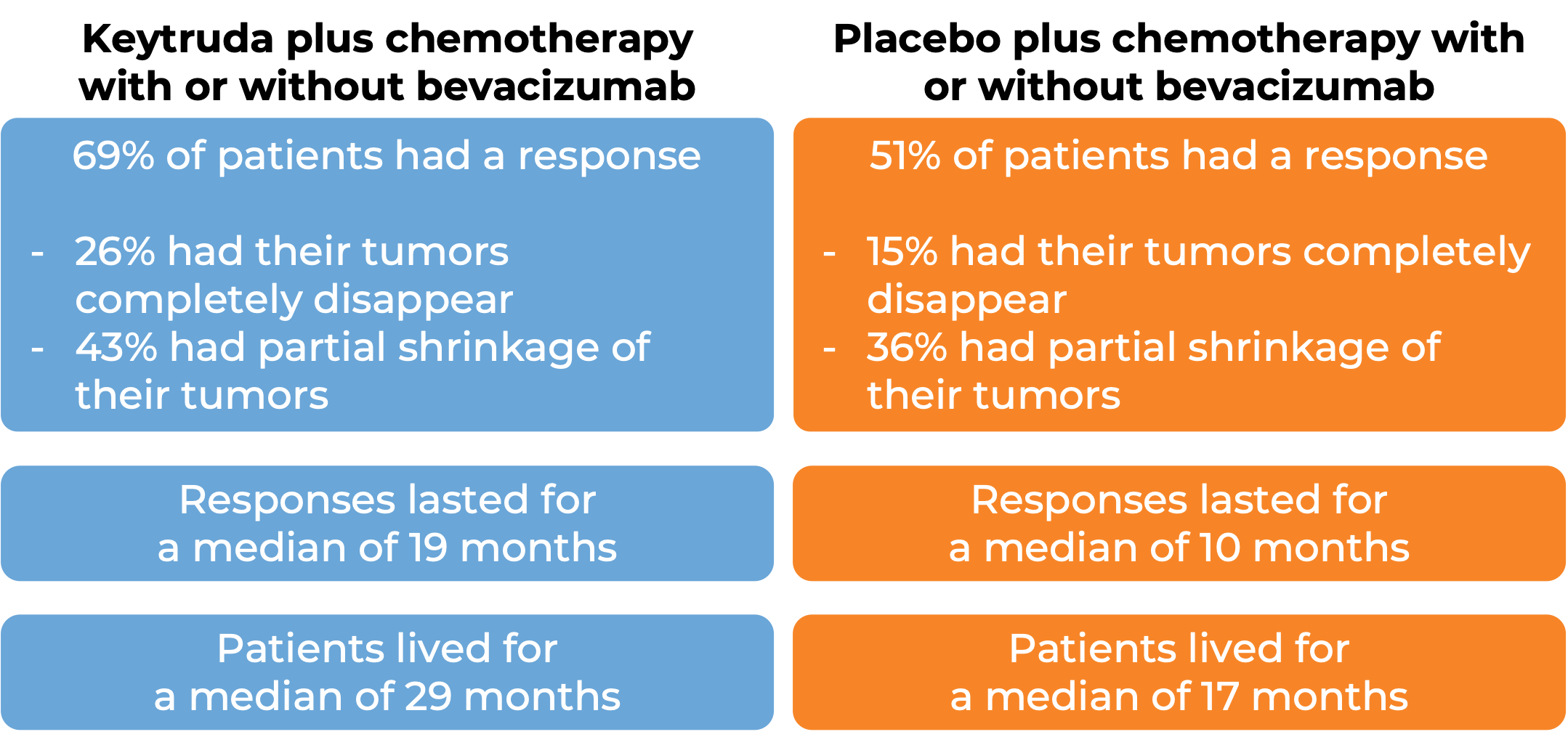
In a clinical trial, 617 patients with cervical cancer that had come back or spread to other parts of the body (metastatic) and who had not yet been treated with chemotherapy (except radiochemotherapy) for their metastatic disease, were treated with either
- Keytruda plus chemotherapy (paclitaxel and cisplatin or carboplatin) with or without bevacizumab (Avastin, Mvasi, Zirabev), OR
- Placebo plus chemotherapy with or without bevacizumab
Among 548 patients whose tumor tested positive for the PD-L1 molecule, 63% of patients received bevacizumab as part of the treatment. At a median follow-up of 22 months:
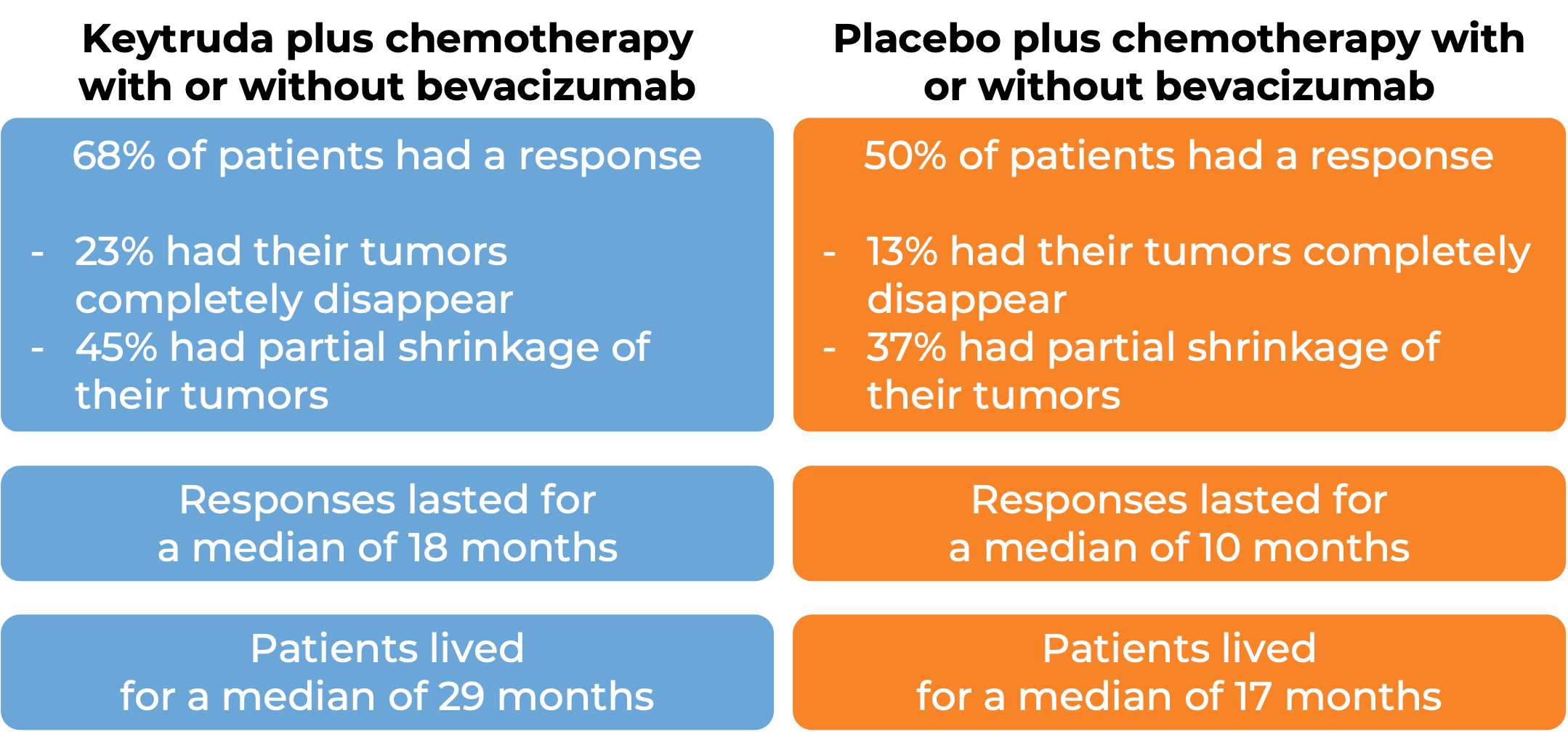
(For the definition of “median”, click HERE.)
Advanced liver cancer
In a clinical trial, 360 patients with hepatocellular carcinoma (liver cancer) caused by a hepatitis B infection, who had been treated before, but not with anti-PD-1/PD-L1 therapy, were treated with Keytruda or placebo.
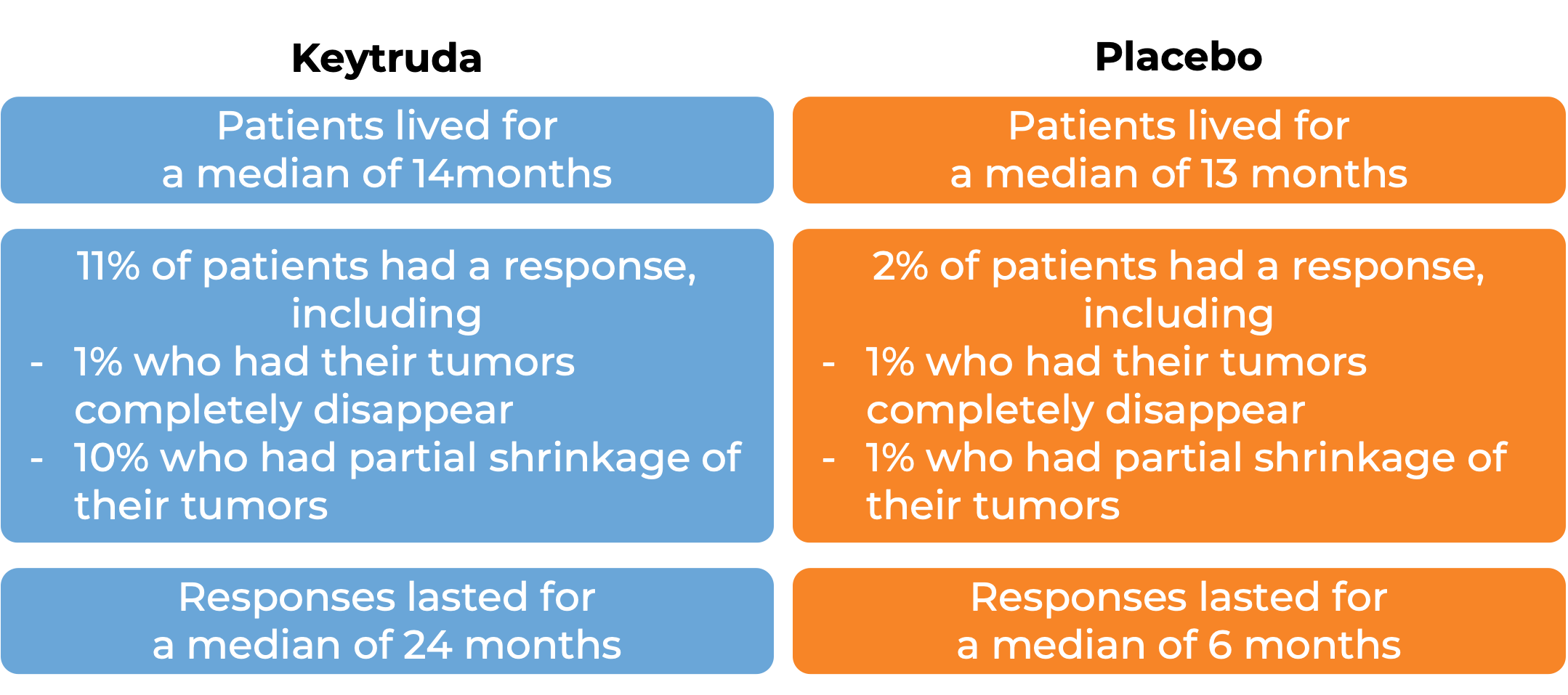
Biliary Tract cancer
In a clinical trial, 1069 patients with locally advanced biliary tract cancer that could not be removed by surgery, or biliary tract cancer that had spread to other parts of the body, and who had not received prior therapy for their advanced disease, were treated with either Keytruda plus cisplatin and gemcitabine chemotherapy or placebo plus cisplatin and gemcitabine chemotherapy. At a median follow-up of 26 months:
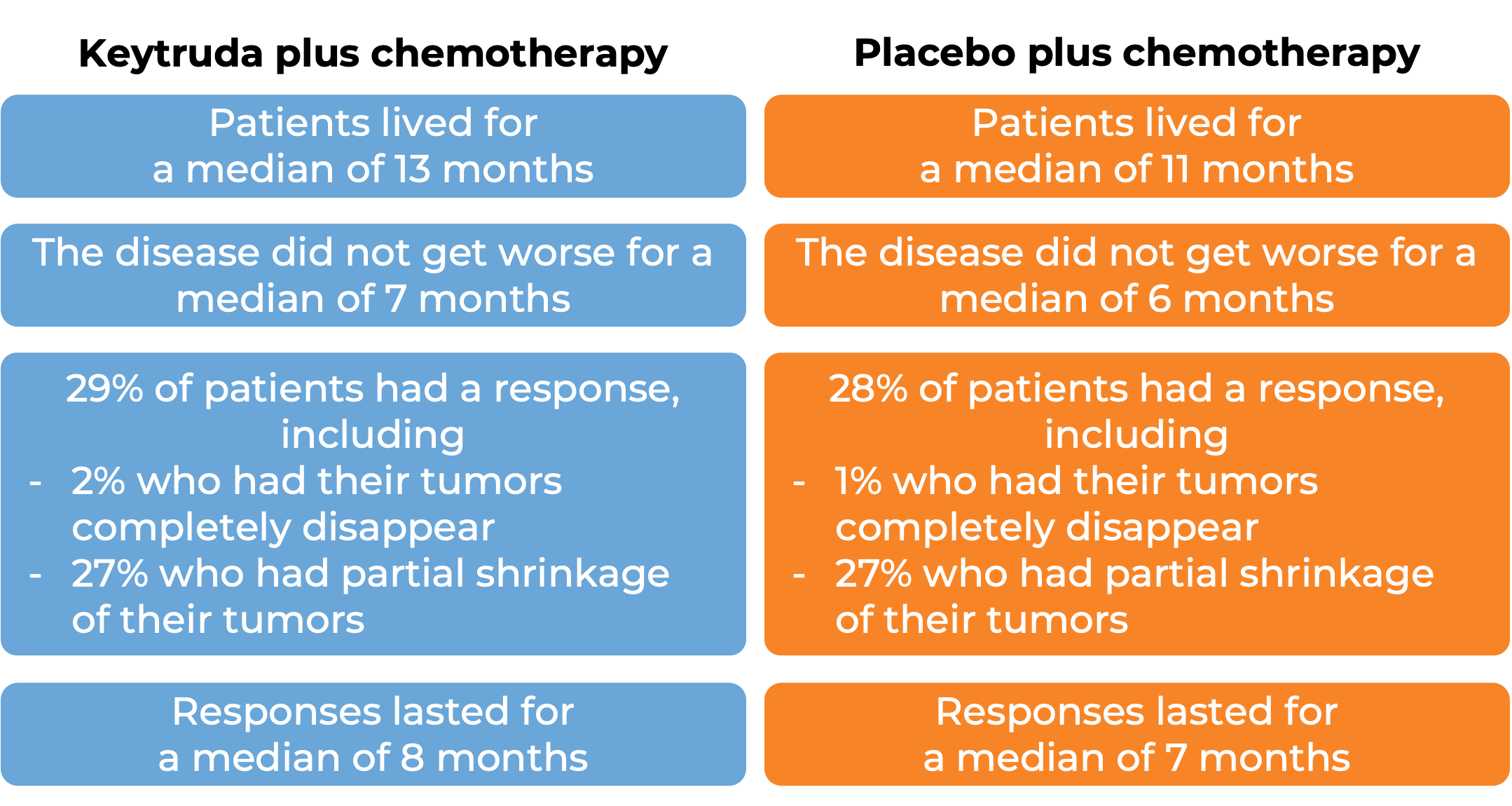
Advanced Merkel cell carcinoma
In a clinical trial, 50 patients with Merkel cell carcinoma that had come back or spread who had not received previous treatment for advanced disease were treated with Keytruda for up to 2 years. The following results were observed:
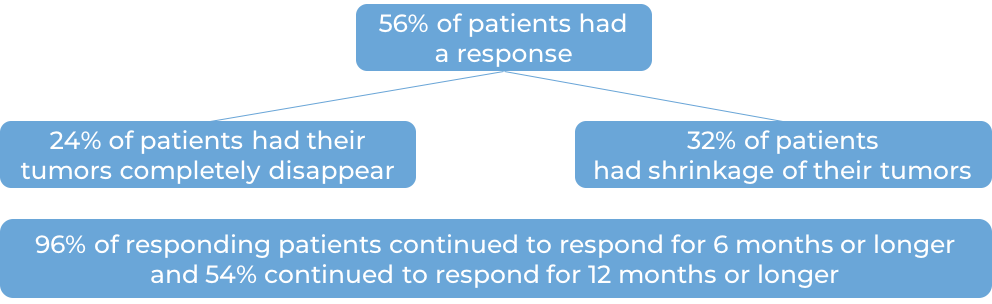
In another clinical trial with 55 patients, similar results were observed.
Advanced kidney cancer
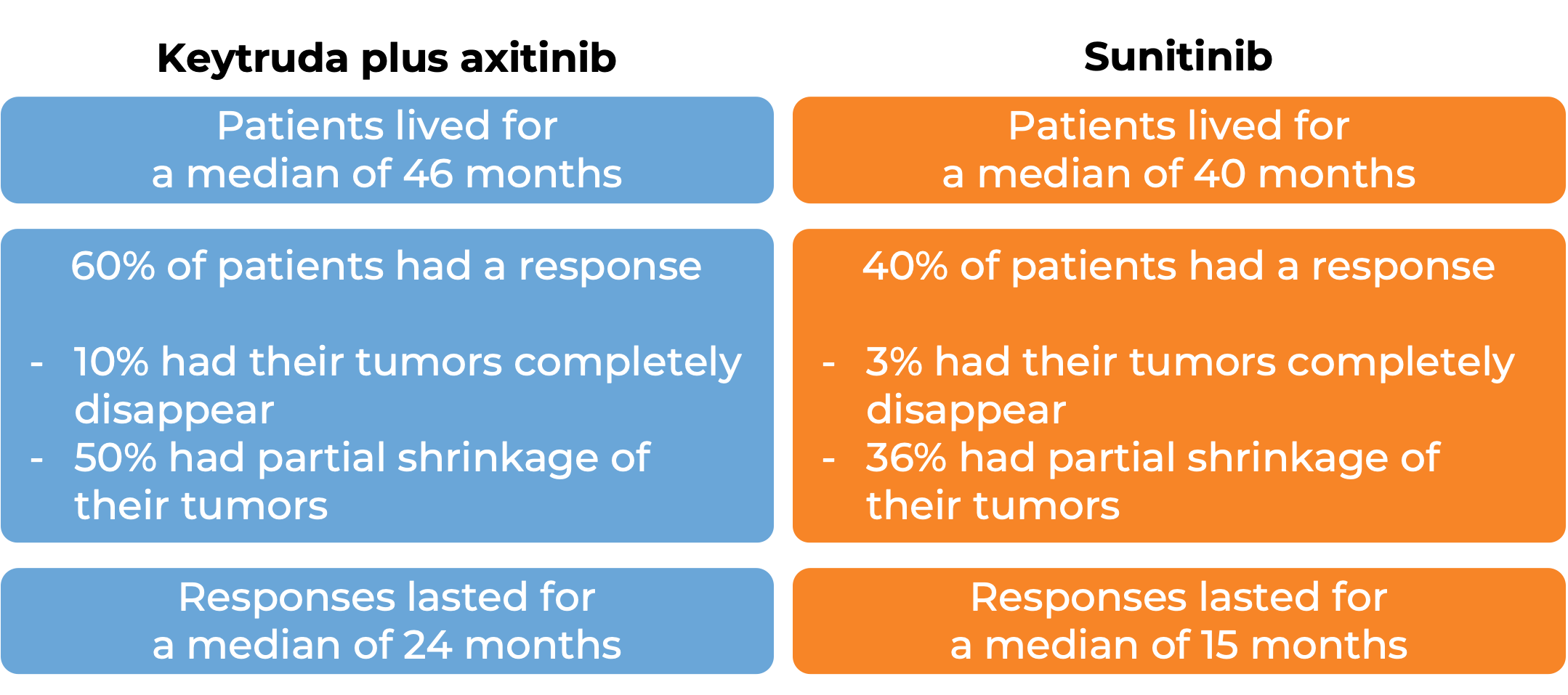
First treatment for advanced kidney cancer
In a clinical trial, 861 patients with advanced renal cell carcinoma who had not received previous treatment for advanced disease were treated with Keytruda plus axitinib or with sunitinib.
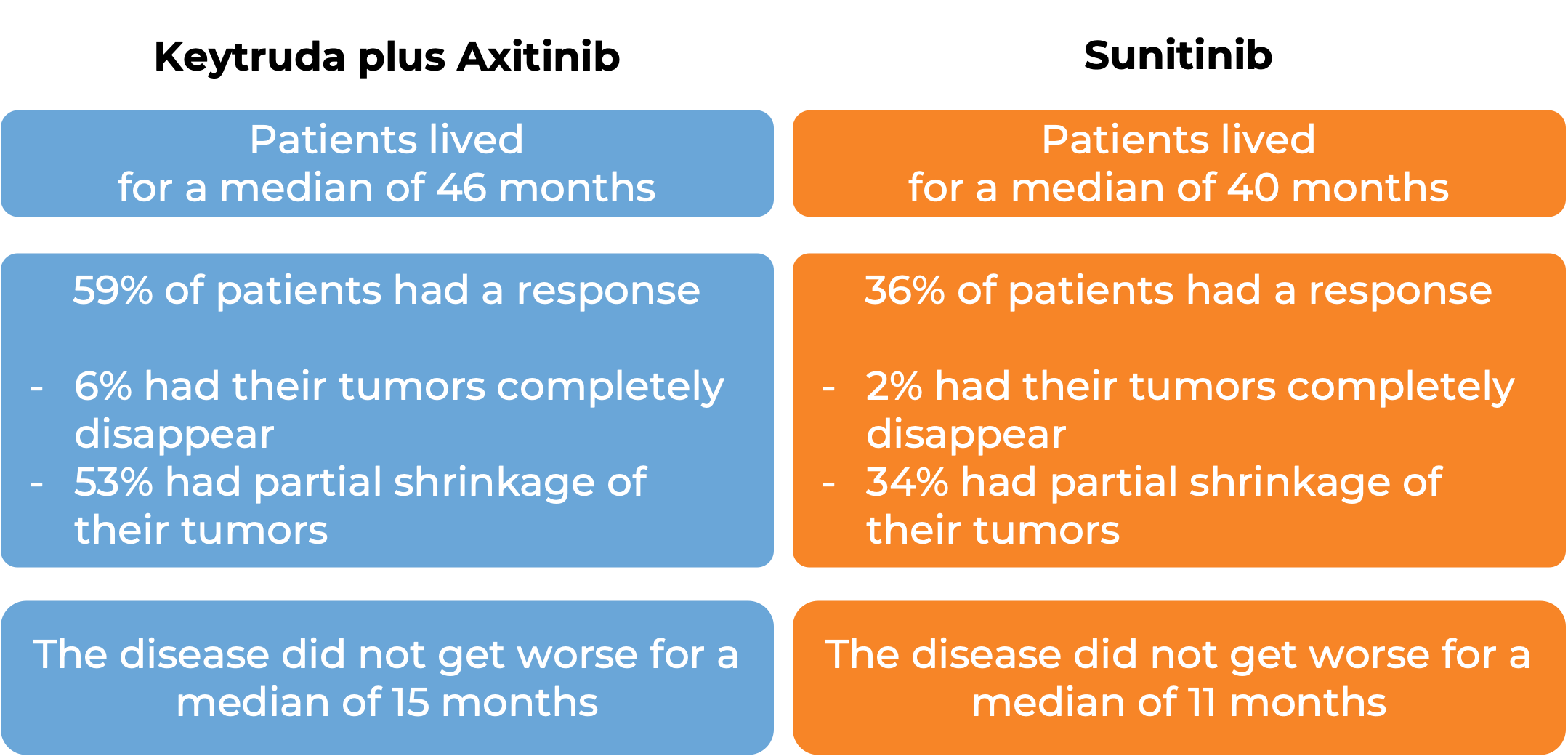
(For the definition of “median”, click HERE.)
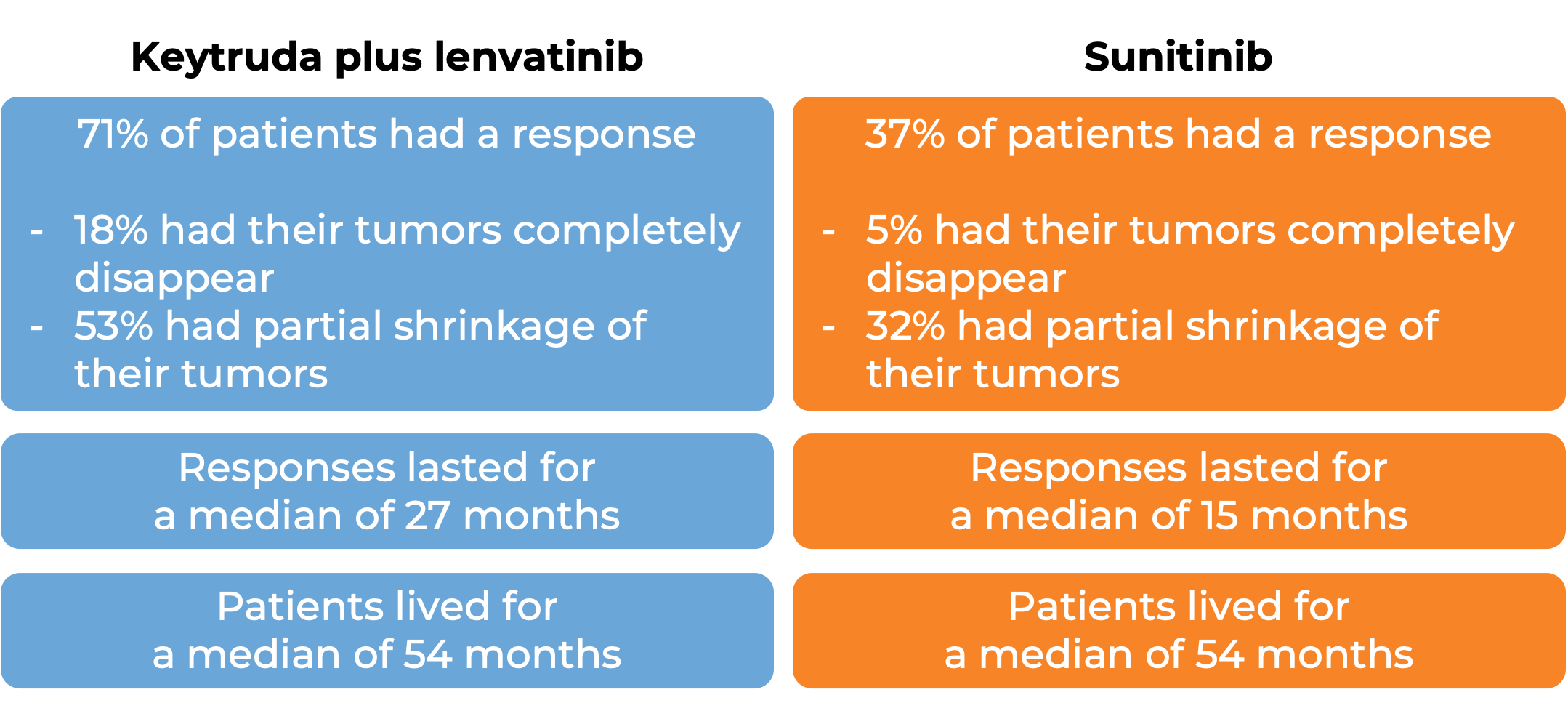
In another clinical trial, 1069 patients with advanced renal cell carcinoma (kidney cancer) were treated with either:
- Keytruda in combination with lenvatinib, OR
- lenvatinib in combination with everolimus, OR
- sunitinib as a first treatment.
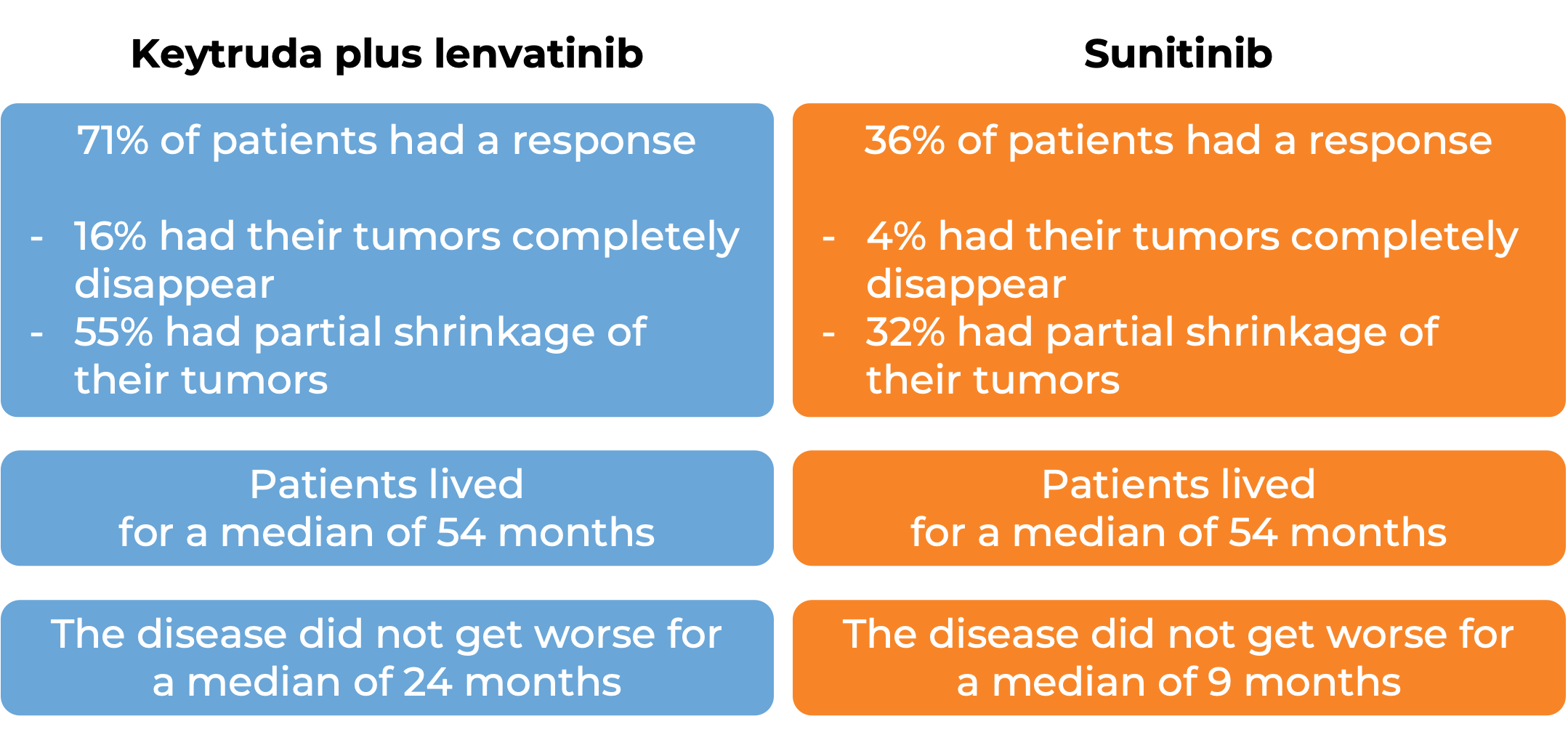
In another clinical trial, 158 patients with renal cell carcinoma (kidney cancer) that had grown locally or had spread to other parts of the body, were treated with Keytruda and lenvatinib as a first treatment.
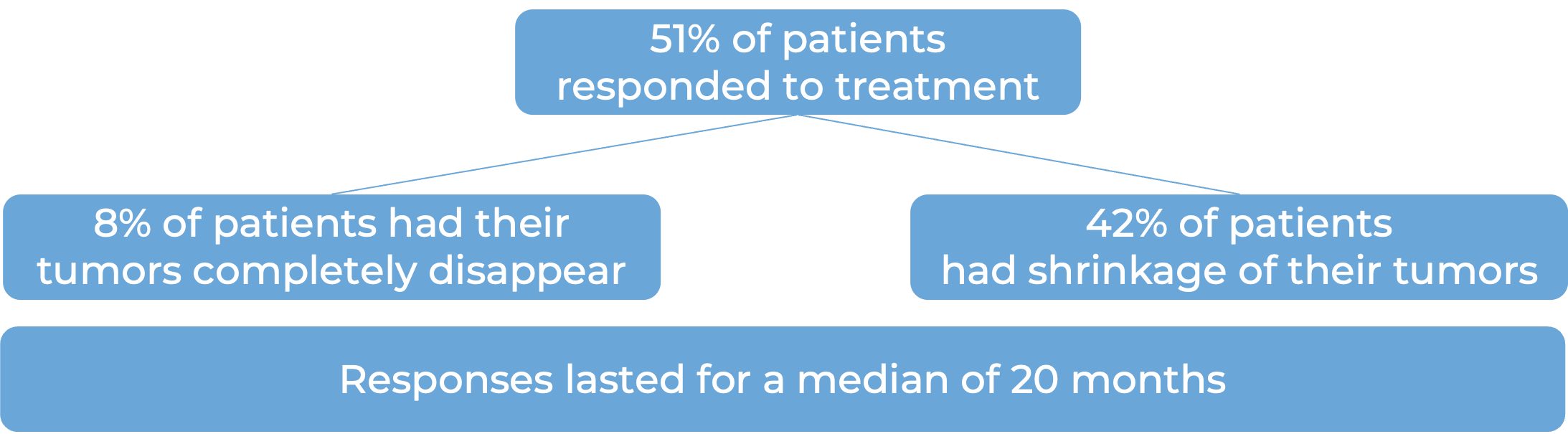
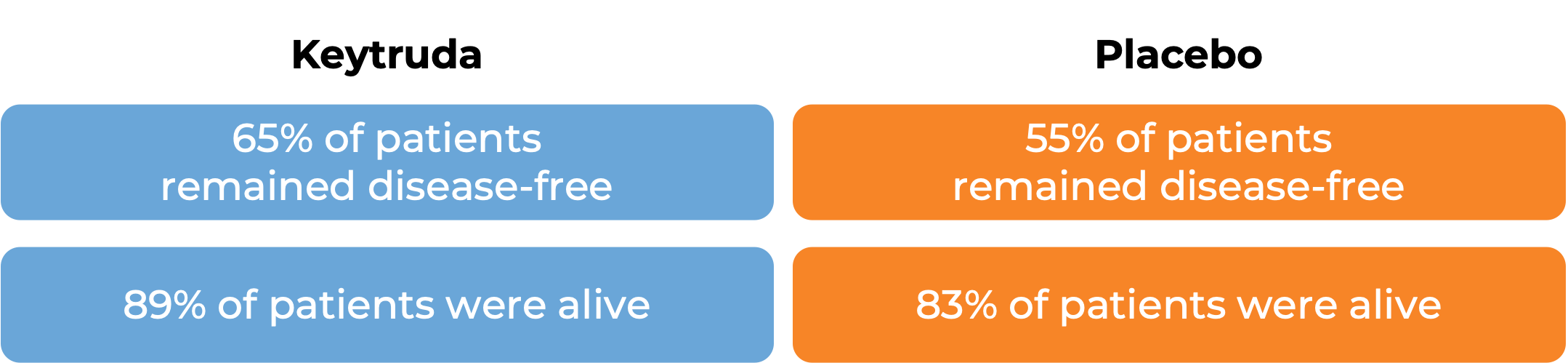
Treatment following surgery for advanced kidney cancer
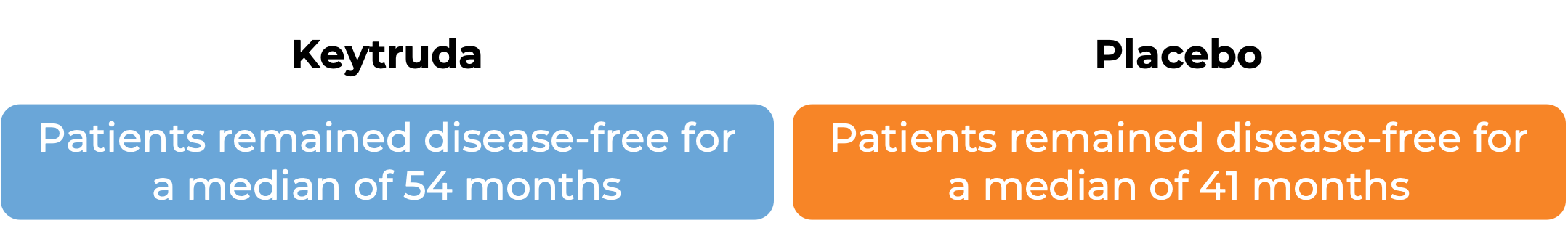
In a clinical trial, 994 patients with renal cell carcinoma (kidney cancer) whose cancer-affected kidney and other tissue the cancer had spread to (metastases) had been partially or completely removed by surgery, and who were at intermediate or high risk for the cancer to come back, were treated with Keytruda or placebo following surgery. After 2 years:

Advanced endometrial carcinoma
Keytruda for advanced endometrial cancer or endometrial cancer that returned
In a clinical trial, 810 patients with advanced endometrial cancer or endometrial cancer that came back, were treated with either:
- Keytruda plus carboplatin and paclitaxel chemotherapy, followed by just Keytruda, OR
- Placebo plus carboplatin and paclitaxel chemotherapy, followed by placebo.
At 12 months and in patients with mismatch repair-deficient (dMMR) endometrial cancer, 74% of patients in the Keytruda group and 38% in the placebo group were alive and did not experience worsening of their disease. In patients with endometrial cancer that was not dMMR, the disease did not get worse for a median of 13 months for patients in the Keytruda group and 9 months for patients in the placebo group.
Endometrial cancer that is not MSI-H or dMMR
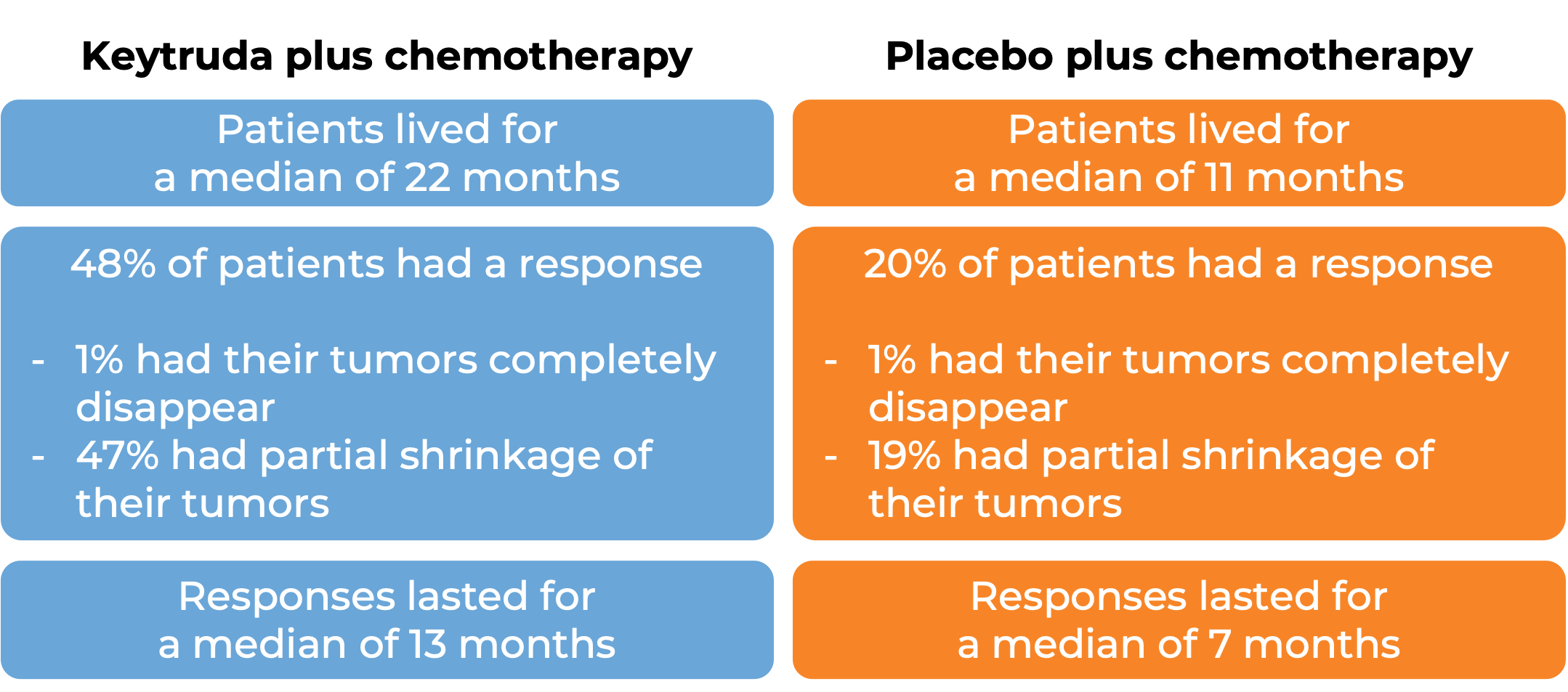
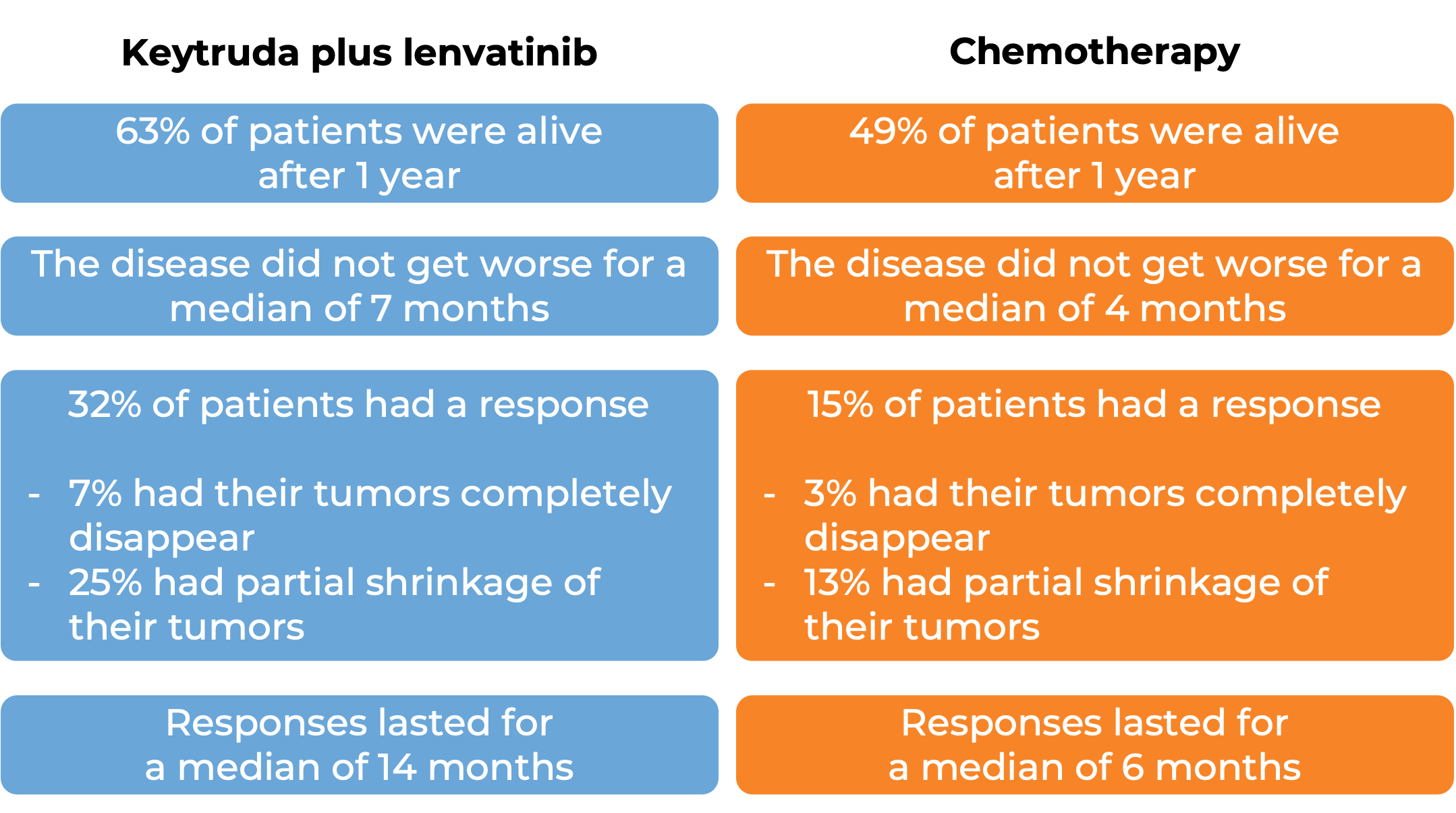
In a clinical trial, 697 patients with metastatic endometrial carcinoma whose cancer was not microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) who had received treatment (including platinum-based chemotherapy), and it did not work or stopped working, were treated with Keytruda plus lenvatinib (Lenvima) or chemotherapy (doxorubicin or paclitaxel) alone. At a median follow-up of 12 and 11 months:
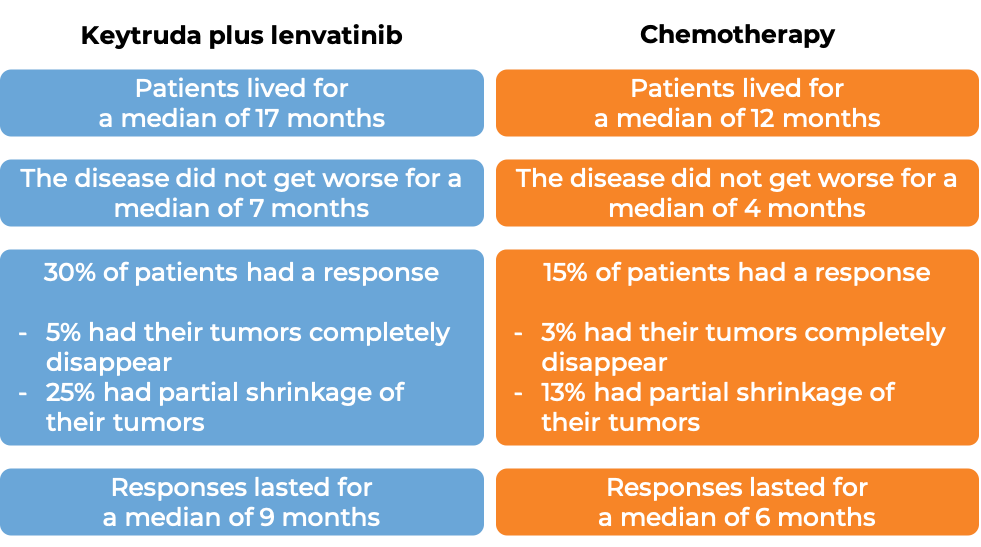
Endometrial cancer that is MSI-H or dMMR
In a clinical trial, 90 patients with previously treated advanced endometrial cancer that was microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR), and whose cancer could not be removed by surgery or had spread to other parts of the body, were treated with Keytruda. At a median follow-up of 16 months:
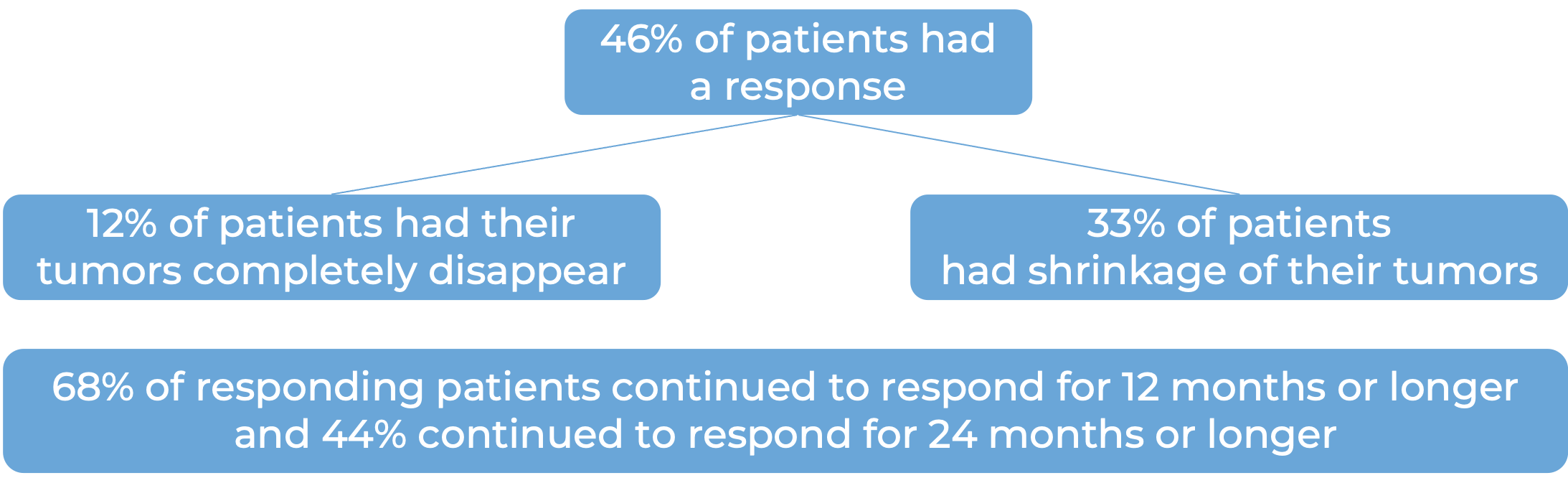
Tumor mutational burden-high (TMB-H) cancer
In a clinical trial, 102 patients with previously treated solid tumors (including small cell lung, cervical, endometrial, anal, vulvar, neuroendocrine, salivary, thyroid, and mesothelioma cancer) whose cancer could not be removed by surgery or had spread, and whose cancer had a high tumor mutational burden, were treated with Keytruda. At a median follow-up of 11 months:
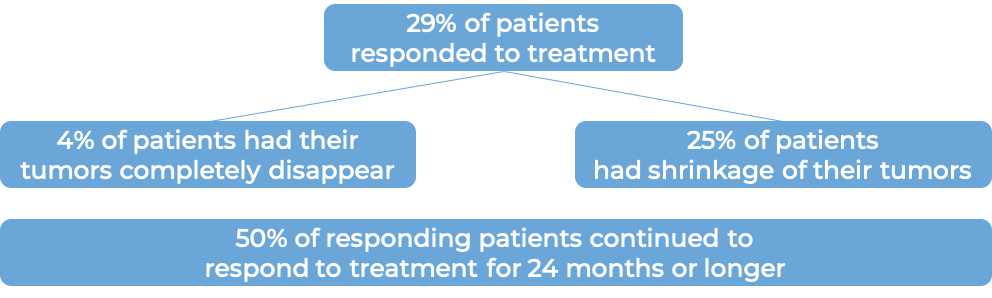
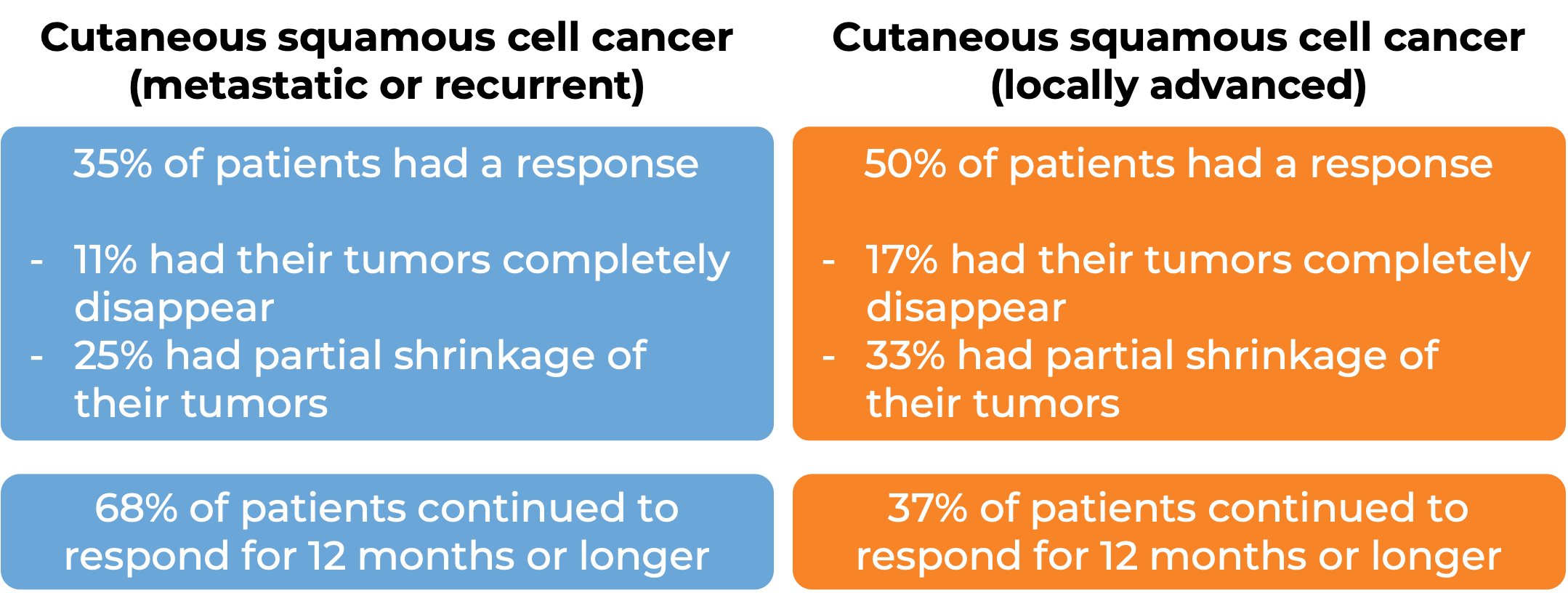
Cutaneous squamous cell carcinoma
In a clinical trial, 105 patients with cutaneous squamous cell carcinoma (a type of skin cancer) that had spread to other parts of the body or had come back (metastatic or recurrent) and 54 patients with cutaneous squamous cell carcinoma that at grown (locally advanced), were treated with Keytruda. At a median follow-up of 24 months for patients with metastatic or recurrent disease and 13 months for patients with locally advanced disease:
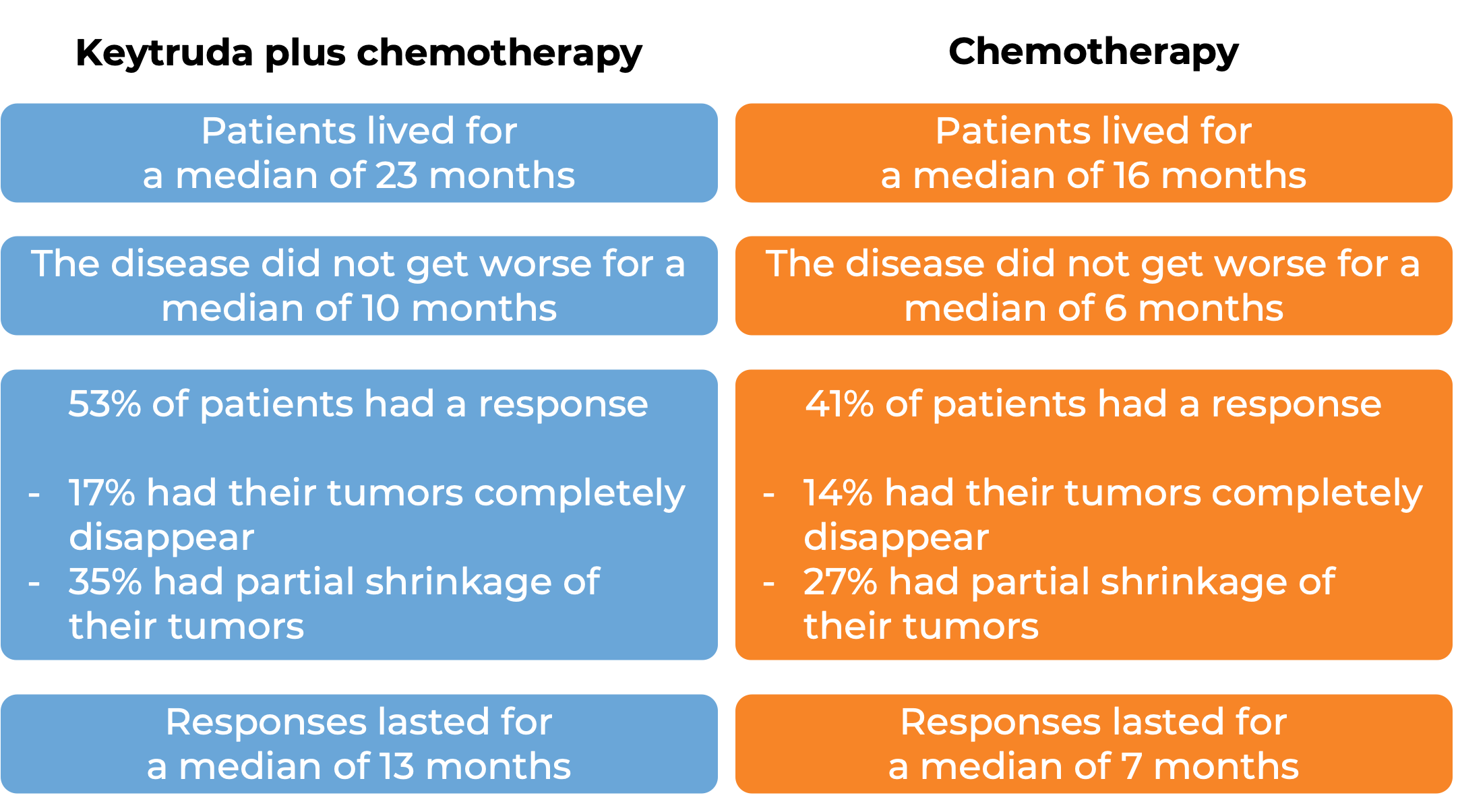
Triple-negative breast cancer
Keytruda for advanced triple-negative breast cancer
In a clinical trial, 847 patients with triple-negative breast cancer that had come back and grown at the original tumor site or had spread to other parts of the body, could not be removed by surgery, and who had not been previously treated with chemotherapy for their advanced disease, were either treated with Keytruda or placebo in combination with chemotherapy (paclitaxel, OR paclitaxel protein-bound, OR gemcitabine and carboplatin). In 323 patients whose tumors were found to produce high levels of PD-L1, the following results were observed at a median follow-up of 44 months:
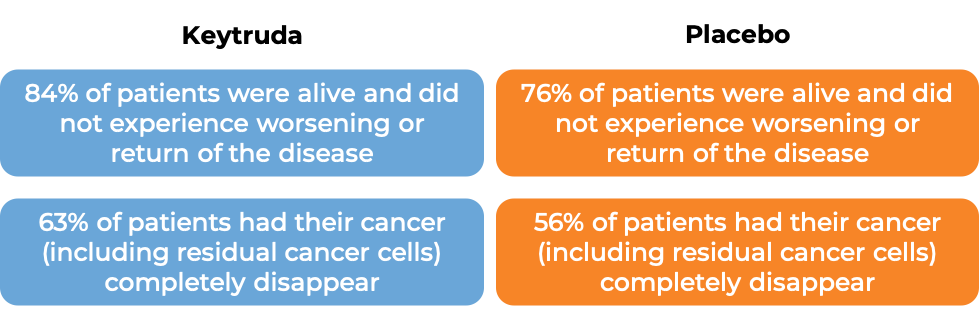
Keytruda for early-stage triple-negative breast cancer
In another clinical trial, 1174 patients with newly diagnosed, previously untreated, early-stage triple-negative breast cancer who were at a high risk for the cancer to become worse, were treated with:
- Keytruda in combination with chemotherapy before surgical removal of all known disease and Keytruda alone after surgery, OR
- Placebo in combination with chemotherapy before surgical removal of all known disease and placebo alone after surgery.
At a median follow-up of 39 months:
What are the side effects?
The most common side effects of Keytruda include fatigue, pain (in the muscles, bones, joints, and stomach area), headache, itching, rash, decreased appetite, diarrhea, constipation, nausea, fever, cough, and shortness of breath.
Keytruda can cause the patient’s T cells to attack healthy cells throughout the body. Because of this, Keytruda can cause side effects that can become serious or life-threatening, and may lead to death. These side effects can happen anytime during treatment or after the treatment has ended. Some of the serious side effects related to Keytruda include inflammation of the lungs, liver, brain, kidneys (which can lead to kidney failure), or colon (which can result in tears or holes in the intestine). Additionally, problems can arise with hormone glands (including thyroid, pituitary, and adrenal glands, as well as the pancreas). Skin rash (which could become severe and life-threatening) and reactions related to the infusion may also occur. Patients should report any symptoms to their healthcare provider who can then initiate actions to limit or reverse the side effects.
Patients may experience other side effects when Keytruda is used in combination with other treatments.
In patients who are treated with Keytruda before or after stem cell transplant, serious and life-threatening transplant-related complications can occur. These complications may arise even if patients have been treated with other types of therapy in between administration of Keytruda and stem cell transplant.
For a more complete list of possible side effects, see the full prescribing information.
Additional information
Manufacturer
Merck
Approval
FDA and EMA
Links to drug websites
Other references
KEYNOTE-335: Randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. Cortes et al. ASCO abstract (2020)
Phase 3 KEYNOTE-775 Trial in Advanced Endometrial Cancer. Westin SN, et al. OncLive (2021)
Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. Eggermont AMM, et al. The New England Journal of Medicine (2018)
Perioperative Pembrolizumab for Early-Stage Non-Small-Cell Lung Cancer. Wakelee H, et al. The New England Journal of Medicine (2023)
Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. Powles T, et al. The New England Journal of Medicine (2024)
Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Rha SY, et al. The Lancet Oncology (2023)
Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Sun JM, et al. The Lancet (2021)
Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Kelley RK, et al. The Lancet (2023)
Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. Rini BI, et al. The New England Journal of Medicine (2019)
Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. Eskander RN, et al. The New England Journal of Medicine (2023)
Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. Makker V, et al. The New England Journal of Medicine (2022)
Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. Cortes J, et al. The New England Journal of Medicine (2022)
Last updated: July 25, 2024