How is this drug name pronounced?
Tisagenlecleucel: TIH-suh-jen-LEK-loo-sel
Kymriah: kim-RY-uh
What cancer(s) does this drug treat?
Kymriah is approved for:
B-cell acute lymphoblastic leukemia (ALL)
Diffuse large B-cell lymphoma (DLBCL)
Follicular lymphoma (FL)
Advanced B-cell acute lymphoblastic leukemia (ALL)
Kymriah is approved for:
- Children and young adult patients up to 25 years of age with B-cell acute lymphoblastic leukemia (ALL) whose cancer did not respond to previous treatment (refractory disease), or responded to two or more previous treatments but then came back (relapsed disease).
Advanced diffuse large B-cell lymphoma (DLBCL)
Kymriah is approved for:
- Adults with diffuse large B-cell lymphoma (DLBCL) whose cancer came back or did not respond (relapsed or refractory, respectively) after two or more previous treatments.
Advanced follicular lymphoma (FL)
Kymriah is approved for:
- Adults with follicular lymphoma (FL) whose cancer came back (relapsed) or did not respond (refractory) after two or more previous treatments.
Limitations of use:
REMS Program: Due to the potential for serious side effects, Kymriah is currently only available through a Risk Evaluation and Mitigation Strategy (REMS) program. Healthcare facilities that plan to treat patients with Kymriah must first qualify for the program, and ensure proper monitoring and management of side effects associated with the drug.
Age: The safety and efficacy of Kymriah in patients with acute lymphoblastic leukemia under the age of 2 and over the age of 65 have not been established. The safety and efficacy of Kymriah in patients with diffuse large B-cell lymphoma or follicular lymphoma under 18 years of age have not been established.
Exclusions: Currently, Kymriah is not manufactured for patients who test positive for active hepatitis B virus (HBV), hepatitis C virus (HCV), or human immunodeficiency virus (HIV).
Primary central nervous system lymphoma: Kymriah is not approved for treatment in patients with central nervous system lymphoma.
Effects on the ability to drive and use machines: Patients are advised to refrain from driving, engaging in hazardous occupations or activities, and operating heavy machinery for at least 8 weeks after receiving Kymriah.
Fertility/Pregnancy/Breastfeeding: The effects of Kymriah on female and male fertility are not known. Kymriah is not recommended for women who are pregnant. The risks associated with Kymriah during breastfeeding are not known, and risk to a breastfed infant cannot be excluded.
Live virus vaccination: Vaccination with live virus vaccines (e.g., chickenpox, measles/mumps/rubella [MMR]) is not recommended for at least 6 weeks prior to the start of lymphodepleting chemotherapy, during Kymriah treatment, and until recovery of the immune cell counts following treatment with Kymriah.
What type of immunotherapy is this?
- CAR T cell therapy
How does this drug work?
- Target: CD19
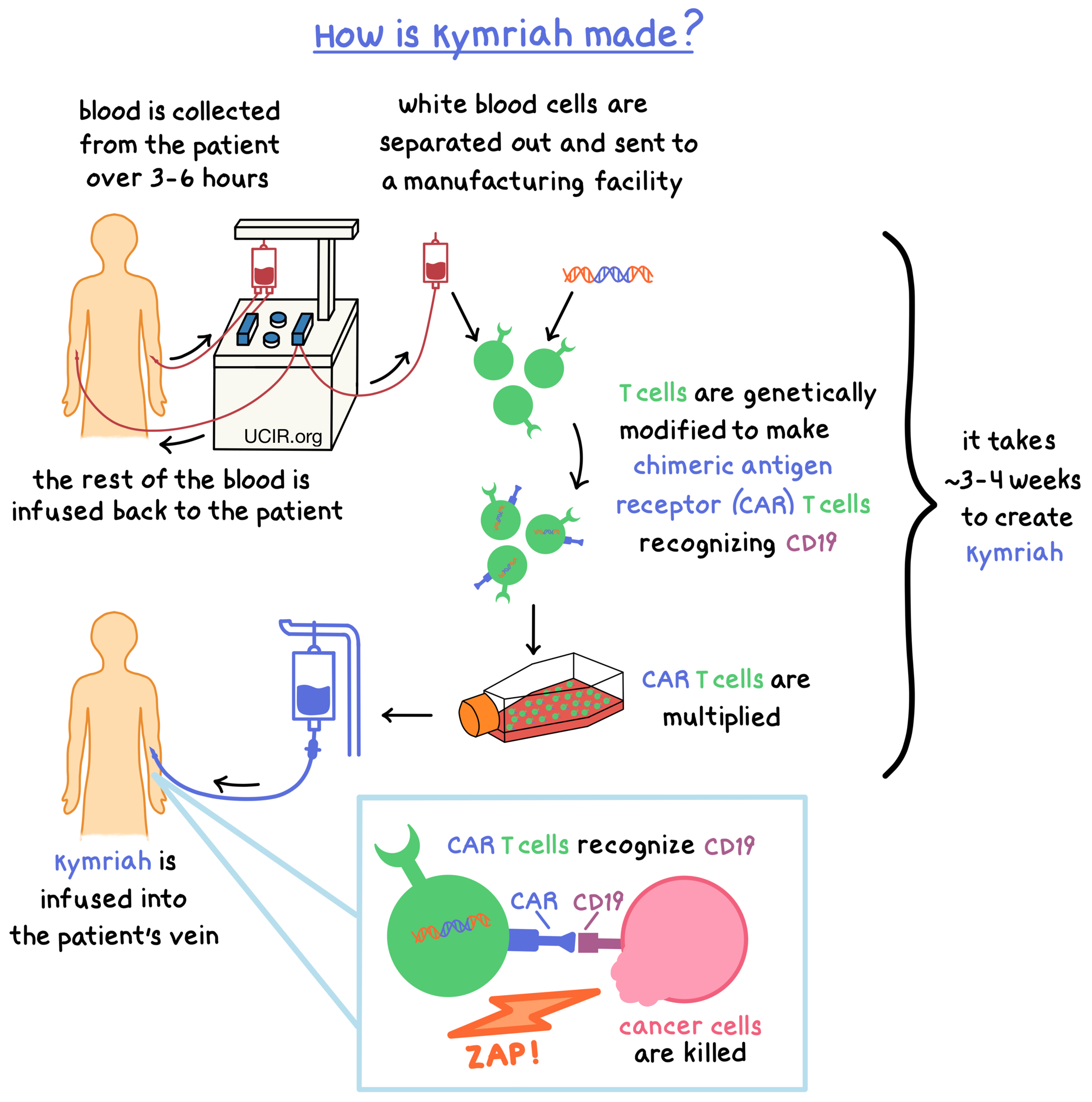
Kymriah is made from the patient’s own T cells (a type of white blood cell). In order to make Kymriah, blood is collected from the patient’s vein – this process usually takes 3 to 6 hours and may need to be repeated. In a process called leukapheresis, the white blood cells (including T cells) are separated from the collected blood, and the rest of the blood is returned to the patient. The collected white blood cells are sent to a specialized manufacturing facility where the patient’s T cells are genetically modified in such a way that they make a protein on their surface called chimeric antigen receptor (CAR). The modified T cells (“CAR T cells”) are multiplied to create millions of CAR T cells.
The CAR on the surface of the modified T cell can attach to a protein called CD19 on the surface of the cancer cell. When CAR attaches to CD19, T cells recognize the cancer cells and kill them. The entire process to create Kymriah from the collected blood could take approximately 3 to 4 weeks.
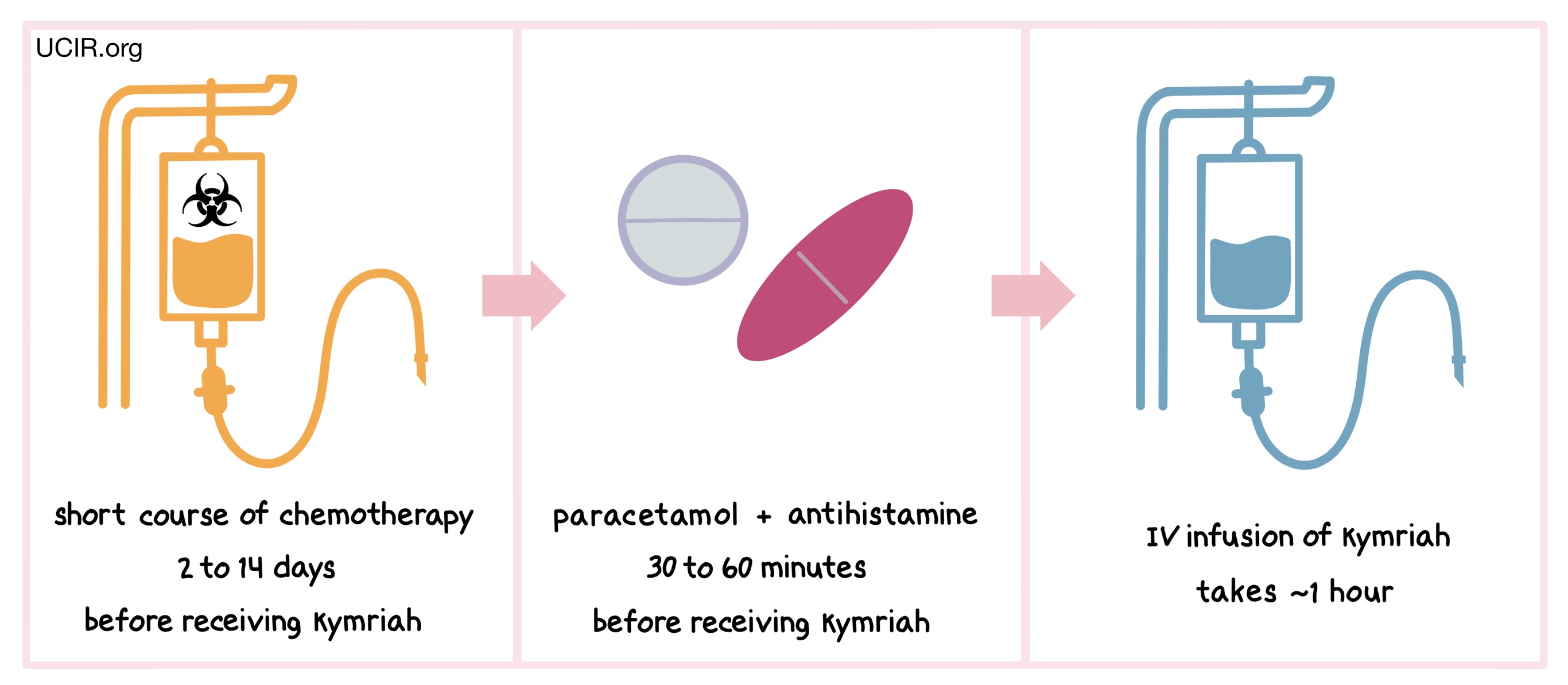
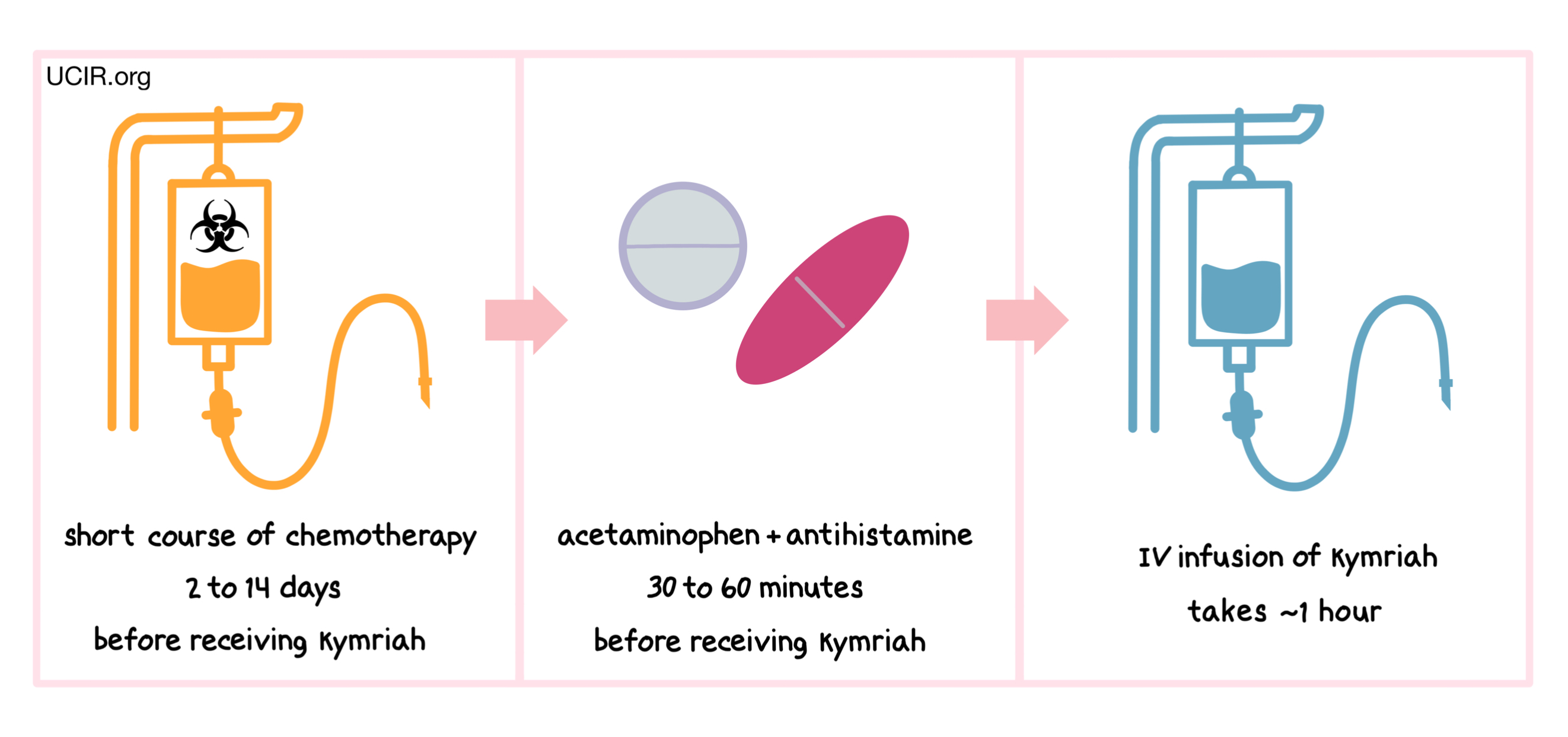
How is this drug given to the patient?
Two to 14 days before receiving Kymriah patients are usually treated with a short course of chemotherapy to reduce the number of white blood cells. The reduction of white blood cells in the patient gives the CAR T cells in Kymriah enough space to multiply and provides the CAR T cells with enough resources needed to persist in the patient longer. About 30 to 60 minutes before receiving Kymriah, patients receive acetaminophen and an antihistamine to reduce the chance of reactions to the infusion.
Patients receive Kymriah through an intravenous (IV) infusion (through a tube in the vein). The administration of Kymriah usually takes about an hour.
Patients should plan to stay close to the treatment location for at least 4 weeks after receiving Kymriah.
What are the observed clinical results?
For:
Advanced B-cell acute lymphoblastic leukemia (ALL)
Advanced diffuse large B-cell lymphoma (DLBCL)
Advanced follicular lymphoma (FL)
It is important to keep in mind that each patient’s actual outcome is individual and may be different from the results found in the clinical studies. In addition, with immunotherapy, sometimes it takes several months for responses to be observed.
Advanced B-cell acute lymphoblastic leukemia (ALL)
In a clinical trial, 63 patients with advanced B-cell ALL were treated with Kymriah. Within 3 months after treatment, 83% of patients had no signs of cancer left, and leukemia cells could no longer be detected with advanced molecular tests (no minimal residual disease).
Advanced diffuse large B-cell lymphoma (DLBCL)
In a clinical trial, 68 patients with advanced DLBCL were treated with Kymriah. 32% of patients had no signs of cancer left, and another 18% saw their cancer shrink but not fully go away within 6 months after treatment.
Advanced follicular lymphoma (FL)
In a clinical trial, 97 patients with previously treated follicular lymphoma were treated with Kymriah. At a median follow-up of 17 months among 94 patients with measurable disease prior to infusion, 69% of patients had no signs of cancer left, and another 17% saw their cancer shrink but not fully go away.
What are the side effects?
Kymriah can lead to side effects, some of which could be long-lasting. The CAR T cells in Kymriah kill cancer cells that have the molecule CD19 on their surface; however, CD19 is also found on the surface of healthy B cells, which can also be killed by Kymriah. The decrease in healthy B cells leads to low levels of antibodies (immunoglobulins) and an increased risk of serious infections. Other possible side effects include low blood cell counts, difficulty breathing, fever, chills, nausea, vomiting, diarrhea, muscle and joint pain, very low blood pressure, dizziness/lightheadedness, headache, fast heartbeat, altered mental state, and serious allergic reactions.
Most patients receiving Kymriah experience serious side effects. Some side effects, such as cytokine release syndrome (CRS), neurological toxicities, and hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS), may be severe or life-threatening. Patients and caregivers receive careful instructions to monitor for signs and symptoms related to CRS and neurological toxicities, and both conditions are managed by the health care provider.
Cytokine release syndrome (CRS)
CRS is caused by a widespread release of molecules called cytokines, which are involved in inflammation and can affect the function of various organs. Cytokines may be released by the CAR T cells in Kymriah or by other immune cells in the patient’s body. Symptoms of CRS include high fever, chills, difficulty breathing, severe muscle or joint pain, severe nausea, vomiting, diarrhea, very low blood pressure, and dizziness or lightheadedness. CRS typically occurs between 1 and 10 days after Kymriah infusion, and the health care provider should be immediately notified if symptoms occur.
Neurological toxicities
Some of the cytokines released during CRS can result in disruption of the blood-brain barrier, leading to the development of neurological toxicities. Symptoms of neurological toxicities include confusion, altered or decreased consciousness, seizures, loss of balance, and difficulty speaking and understanding. Neurological toxicities typically occur about 7 days after Kymriah infusion, but could occur 8 weeks or later after infusion.
Hemophagocytic lymphohistiocytosis/Macrophage activation syndrome (HLH/MAS)
Some of the cytokines released during cytokine release syndrome can result in an excessive activation of the immune system, causing HLH/MAS. In HLH/MAS, immune cells such as lymphocytes (in HLH) and macrophages (in MAS) begin attacking and destroying healthy tissues, causing widespread tissue damage and inflammation. Symptoms of HLH/MAS typically occur within 3-18 days of receiving Kymriah, and include low blood pressure, low oxygen levels in the blood, altered organ function, and multiple organ failure.
Interference with lab tests for HIV infection
Some lab tests for HIV infection may yield false-positive results in patients who have received Kymriah.
For a more complete list of possible side effects, see the full prescribing information.
Additional information
Manufacturer
Novartis
Approval
FDA and EMA
Links to drug websites
Other references
- Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. Maude SL, Laetsch TW, et al. The New England Journal of Medicine (2018)
- Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. Schuster SJ, Bishop MR, et al. The New England Journal of Medicine (2019)
- Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Fowler NH, Dickinson M, et al. Nature Medicine (2021)
Last updated: January 16, 2023