mRNA vaccines: from cancer immunotherapy to COVID-19
2021-07-14
When COVID-19 took the world by storm in late 2019, the race to create an effective vaccine began almost immediately. The sheer urgency of the situation propelled massive research efforts and allowed for a handful of the usual regulatory hurdles to be cast aside, and with that, the pace of vaccine development far surpassed anything the world had ever seen. From the potential vaccine pool, two frontrunners emerged – one from Pfizer-BioNTech and one from Moderna. Interestingly, both were from a seemingly novel class of vaccines that use mRNA rather than conventional proteins. While many were surprised by this non-traditional approach, those familiar with the field of cancer immunotherapy were not so surprised.
While the new mRNA vaccines for COVID-19 are the first of their kind to gain approval for commercial use, the concept and the vaccine platform itself are not particularly new. With the discovery of mRNA molecules in 1961, investigation began into their potential for medical use. In general, RNA serves as a set of instructions – sort of a photocopy of DNA – that contains the immediate instructions for producing proteins. This makes it an incredibly versatile tool. Using mRNA, researchers have investigated protein replacement therapies, allergy treatments, and even vaccines for HIV, influenza, and other infectious diseases. By and large, though, mRNA vaccines have been most investigated as a potential treatment of cancer.
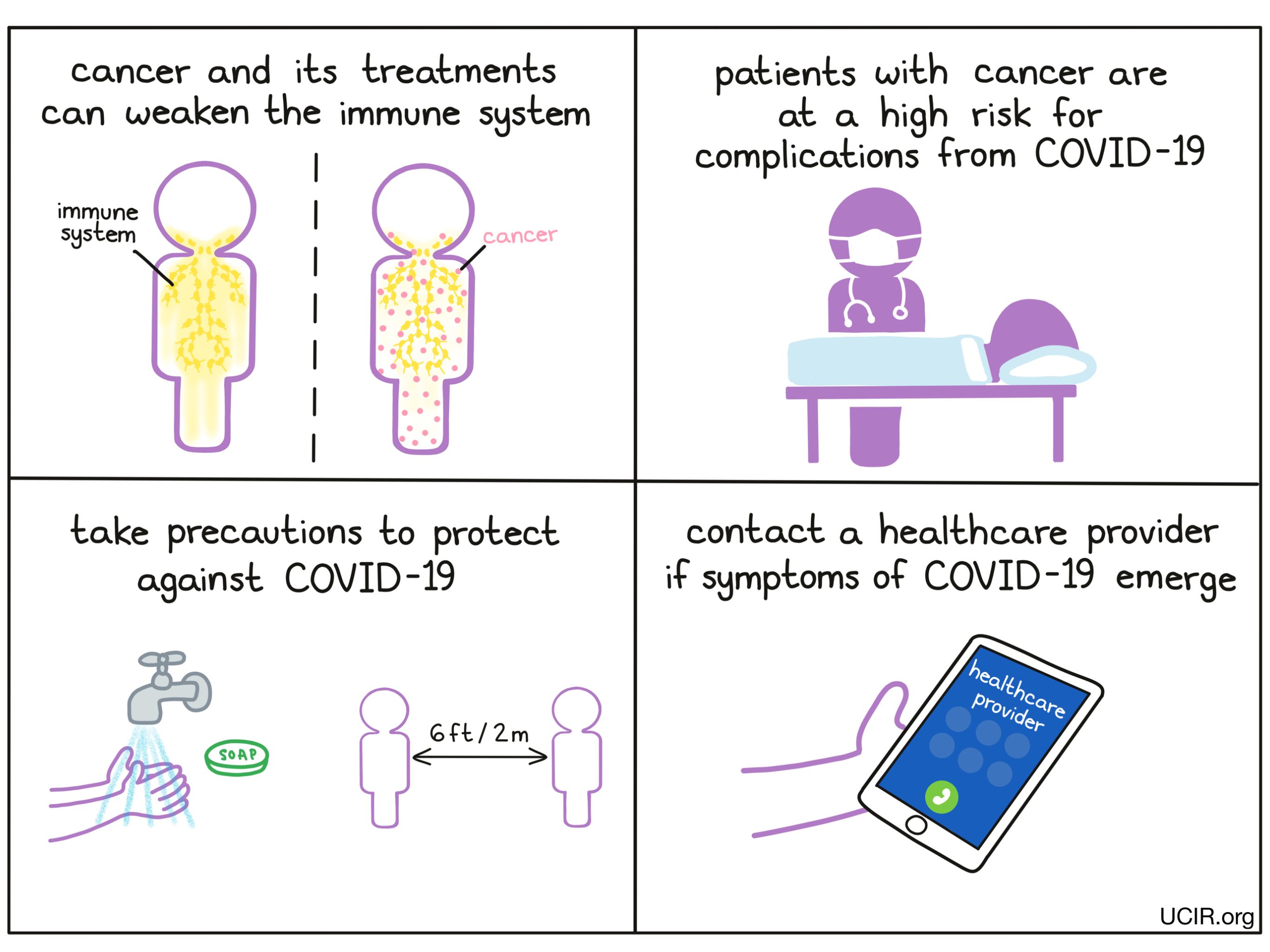
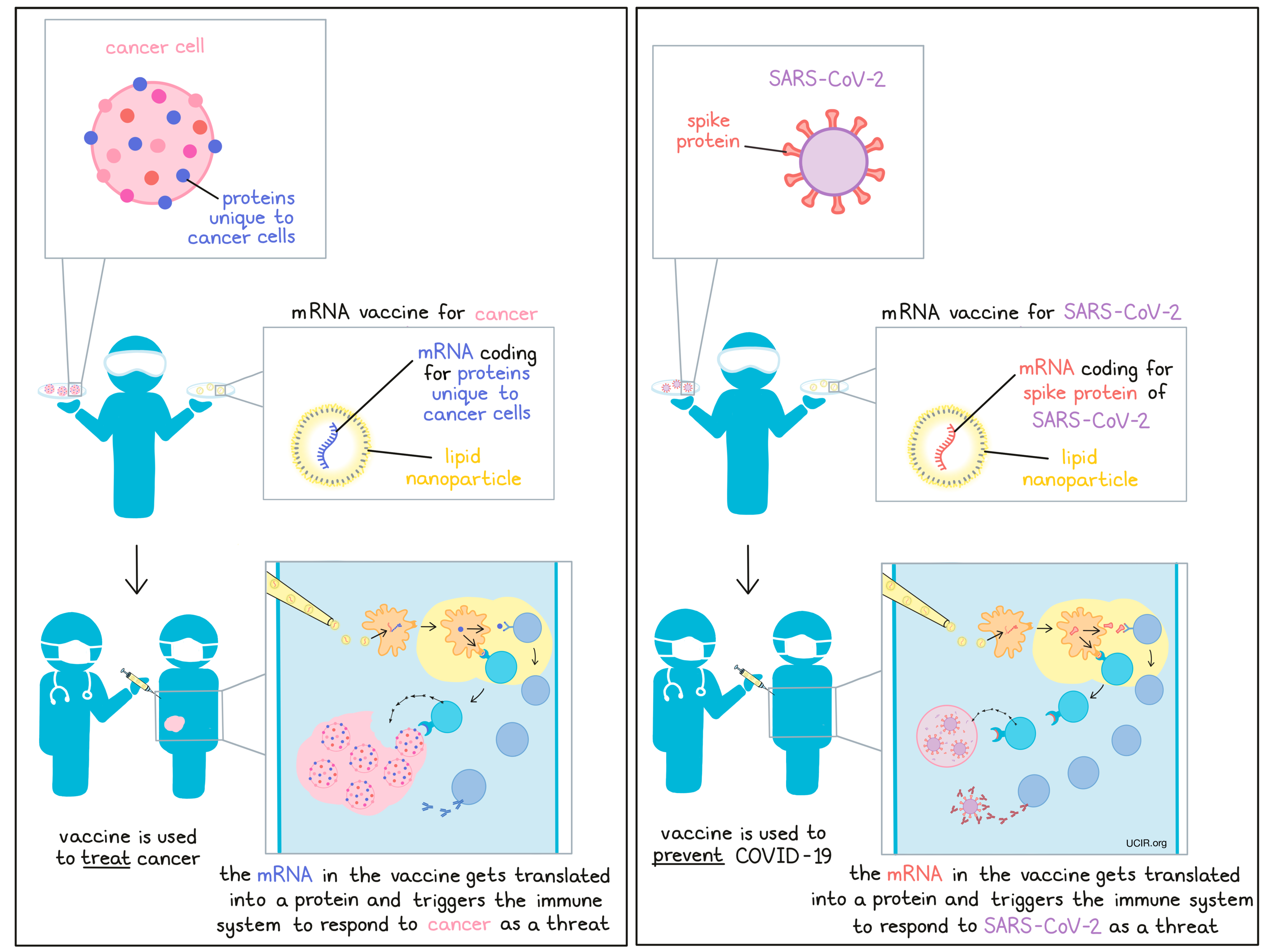
When cancer cells form in the body, they begin to take on new qualities and produce proteins that are not produced by most normal, healthy cells. Sometimes DNA mutations in cancer cells cause them to produce entirely new protein molecules not found anywhere else in the body. These out-of-place molecules, called antigens, distinguish cancerous cells from normal, healthy ones, and can catch the immune system’s attention, but often not enough to allow the immune system recognize the cancer as the threat that it is.
The idea behind personalized cancer vaccines is to identify the unique mutations and molecules in the cancer of an individual patient and formulate them into a vaccine that triggers a strong immune response against them, turning the immune system against the cancer and triggering an immune attack. For cancer vaccine development, mRNA serves as a particularly useful platform, as it can be used to cheaply and quickly make almost any protein, and, when delivered from outside the body, it naturally triggers immune responses to whatever protein is encoded.
The first vaccination using mRNA encoding cancer antigens occurred in 1995 in mice, and a year later, researchers showed that a type of immune cell, dendritic cells, could effectively take up and translate mRNA. Whole dendritic cells could also be used as vaccines to stimulate immune responses and inhibit tumor growth. In the years since, the field has made slow but significant progress. Researchers have worked to improve the ability of mRNA to effectively enter cells, and have modified it to improve its stability and its ability to be translated into the intended protein. Further modifications have been made to fine-tune it’s structure so that the optimal immune responses are triggered, in order to stimulate strong, long-lasting immunity.
In recent years, several studies using mRNA or dendritic cells exposed to mRNA have entered clinical development and have proven both safe and feasible in patients, with the promise of efficacy on the horizon. As trials have moved forward with the potential to develop real-world therapies, work has ramped up to increase the speed at which these vaccines can be developed and the amounts that can be produced.
In 2014, Uğur Şahin, CEO of BioNTech and a long-time leader in the field of mRNA-based therapeutics, co-wrote a review on the state of mRNA-based therapeutics, and while he highlighted cancer immunotherapy as “the only field in which clinical testing and industrialization of the manufacturing of mRNA drugs is at an advanced stage” he also made an interesting note that “beyond applications for cancer immunotherapy, mRNA-based vaccine development may also create opportunities to manage newly emerging pandemics.”
Despite the fact that few mRNA vaccines for infectious diseases had previously entered clinical development, Şahin and other mRNA researchers recognized the versatility, robustness, and relatively low cost of mRNA vaccines, and when COVID-19 struck, they were uniquely poised to rise to the challenge of developing a new vaccine to fight it.
Building upon research from a previous coronavirus outbreak, Severe Acute Respiratory Syndrome (commonly known as SARs), which was active from 2002-2004, researchers were able to quickly identify the coronavirus spike protein as a suitable target that can be recognized by the immune system. They then generated mRNA that codes for the spike protein, and packaged it into small, stable droplets, or “nanoparticles”, to be used as a vaccine. When injected into the muscle, this vaccine delivers the mRNA to nearby lymph nodes, where it gets translated and elicits neutralizing antibodies and T cell responses against the spike protein, and the mRNA is broken down naturally. After two doses, a “prime” and a “boost”, this highly effective vaccine protected up to 95% of patients from developing COVID-19.
As clinical trials were underway, companies began mass production of the vaccines in order to have an ample supply at the ready. The production of these vaccines, which consist of mRNA loaded into a nanoparticle, is fast and scalable. After only 11 months in production, an mRNA vaccine was authorized for emergency use for the first time, and vaccinations began around the globe.
What seems like rapid progress in the development for a vaccine to prevent COVID-19, is in fact the result of decades-long strong research, much of which was in the field of cancer immunotherapy. With the real-world success of the COVID-19 vaccine and the focus on public health, many in the field of medical research are hoping to ride the wave.
In a letter recently published in Cancer Cell, researchers highlighted the unprecedented scale of clinical trials for the COVID-19 vaccines. With a shared sense of responsibility, volunteers came out in droves to participate in clinical trials and contribute to the greater good. While cancer research could benefit from this same level of enthusiastic participation, the slower pace of progress in the field does not draw the same sense of urgency or enthusiasm. Going forward, it is important to articulate the value of clinical trials to patients with cancer. Simplifying clinical trial protocols, increasing transparency, and lowering barriers to entry could serve to attract broader participation in clinical trials, and recruit a more diverse and representative pool of patients, as disparities in healthcare have become even more apparent during the COVID-19 pandemic.
Research in cancer immunotherapy fueled development of a vaccine for COVID-19, and hopefully, the rapid development and real-world success of those vaccines can propel research in other fields as well. Cancer is an ongoing threat and a public health crisis in its own right, and riding the wave of interest and participation in health and medicine could help drive the next great breakthrough in this field and the hope of a better future.
by Lauren Hitchings